Acumen Pharmaceuticals to Deliver Late-Breaking Presentation at 17th Annual Clinical Trials on Alzheimer’s Disease (CTAD) Conference
23 Octobre 2024 - 2:00PM

Acumen Pharmaceuticals, Inc. (NASDAQ: ABOS), a clinical-stage
biopharmaceutical company developing a novel therapeutic that
targets soluble amyloid beta oligomers (AβOs) for the treatment of
Alzheimer’s disease (AD), today announced that it will present
a late-breaking presentation featuring insights from its
participant screening approach used in the ongoing Phase 2
ALTITUDE-AD clinical trial evaluating sabirnetug (ACU193), at the
17th Annual Clinical Trials on Alzheimer’s Disease (CTAD)
conference taking place in Madrid, Spain, and online from Oct. 29 –
Nov., 1, 2024.
Acumen will provide updated data on how it uses
a validated research-use plasma pTau217 assay to screen potential
participants in ALTITUDE-AD. Phosphorylated tau at position 217 is
a biomarker that indicates AD pathology. The plasma pTau217 assay
is being used as an initial screening tool to identify people who
qualify for additional amyloid testing to determine eligibility for
the ALTITUDE-AD trial.
Late-Breaking
Presentation:
LB11 – ALTITUDE-AD: Use of a Validated
p-tau217 Assay to Screen Potential Participants in an Ongoing
Randomized, Double-Blind, Placebo-Controlled Phase 2 Study of
Sabirnetug for Participants with Early Alzheimer's
DiseaseDate/Time: Thursday, Oct. 31,
2024, 9:30 a.m. CETLocation: Auditorium, Madrid
Marriott Auditorium Hotel and Conference Center (Madrid,
Spain)Presenter: Todd Feaster, Psy.D., Senior
Clinical Research Scientist, Acumen Pharmaceuticals
About Sabirnetug (ACU193)
Sabirnetug (ACU193) is a humanized monoclonal
antibody (mAb) discovered and developed based on its selectivity
for soluble amyloid beta oligomers (AβOs), which are a highly toxic
and pathogenic form of Aβ, relative to Aβ monomers and amyloid
plaques. Soluble AβOs have been observed to be potent neurotoxins
that bind to neurons, inhibit synaptic function and induce
neurodegeneration. By selectively targeting toxic soluble AβOs,
sabirnetug aims to address the hypothesis that soluble AβOs are an
early and persistent underlying cause of the neurodegenerative
process in Alzheimer’s disease (AD). Sabirnetug has been granted
Fast Track designation for the treatment of early AD by the U.S.
Food and Drug Administration and is currently being evaluated in a
Phase 2 study in patients with early AD.
About ALTITUDE-AD (Phase 2)
Initiated in 2024, ALTITUDE-AD is a Phase 2,
multi-center, randomized, double-blind, placebo-controlled clinical
trial designed to evaluate the efficacy and safety of sabirnetug
(ACU193) infusions administered once every four weeks in slowing
cognitive and functional decline as compared to placebo in
participants with early Alzheimer's disease. The study will enroll
approximately 540 individuals with early Alzheimer’s disease (mild
cognitive impairment or mild dementia due to AD). The global study
is currently ongoing at multiple investigative sites located in the
United States, Canada, UK, and the European Union. More information
can be found on www.clinicaltrials.gov, NCT identifier
NCT06335173.
About Acumen Pharmaceuticals,
Inc.
Acumen Pharmaceuticals is a clinical-stage
biopharmaceutical company developing a novel therapeutic that
targets toxic soluble amyloid beta oligomers (AβOs) for the
treatment of Alzheimer’s disease (AD). Acumen’s scientific founders
pioneered research on AβOs, which a growing body of evidence
indicates are early and persistent triggers of Alzheimer’s disease
pathology. Acumen is currently focused on advancing its
investigational product candidate, sabirnetug (ACU193), a humanized
monoclonal antibody that selectively targets toxic soluble AβOs, in
its ongoing Phase 2 clinical trial ALTITUDE-AD (NCT06335173) in
early symptomatic Alzheimer’s disease patients, following positive
results in its Phase 1 trial INTERCEPT-AD. The company is
headquartered in Newton, Mass. For more information, visit
www.acumenpharm.com.
Investors: Alex Braunabraun@acumenpharm.com
Media:Jon YuICR Westwicke
AcumenPR@westwicke.com
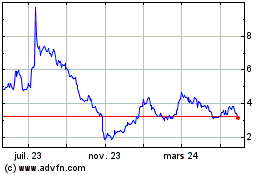
Acumen Pharmaceuticals (NASDAQ:ABOS)
Graphique Historique de l'Action
De Jan 2025 à Fév 2025
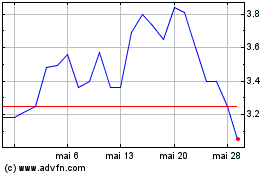
Acumen Pharmaceuticals (NASDAQ:ABOS)
Graphique Historique de l'Action
De Fév 2024 à Fév 2025