0001314102false00013141022024-05-082024-05-08
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
|
Date of Report (Date of earliest event reported): May 08, 2024 |
EyePoint Pharmaceuticals, Inc.
(Exact name of Registrant as Specified in Its Charter)
|
|
|
|
|
Delaware |
000-51122 |
26-2774444 |
(State or Other Jurisdiction
of Incorporation) |
(Commission File Number) |
(IRS Employer
Identification No.) |
|
|
|
|
|
480 Pleasant Street |
|
Watertown, Massachusetts |
|
02472 |
(Address of Principal Executive Offices) |
|
(Zip Code) |
|
Registrant’s Telephone Number, Including Area Code: (617) 926-5000 |
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
☐Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
☐Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
☐Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
☐Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
|
|
|
|
Title of each class
|
|
Trading
Symbol(s) |
|
Name of each exchange on which registered
|
Common Stock, par value $0.001 |
|
EYPT |
|
The Nasdaq Global Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company ☐
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Item 2.02 Results of Operations and Financial Condition.
On May 8, 2024, EyePoint Pharmaceuticals, Inc. (the “Company”) issued a press release announcing its financial results for the quarter ended March 31, 2024 and certain other information. A copy of the press release is furnished as Exhibit 99.1 hereto.
Item 9.01 Financial Statements and Exhibits.
(d) Exhibits.
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
|
|
|
|
|
|
EYEPOINT PHARMACEUTICALS, INC. |
|
|
|
|
Date: |
May 8, 2024 |
By: |
/s/ George O. Elston |
|
|
|
George O. Elston
Executive Vice President and Chief Financial Officer |
Exhibit 99.1
EyePoint Pharmaceuticals Reports First Quarter 2024 Financial Results and Highlights Recent Corporate Developments
–Company on track to initiate the Phase 3 LUGANO pivotal non-inferiority trial of DURAVYU™ in wet AMD in 2H 2024 –
– $299 million of cash and investments on March 31, 2024, with cash runway through topline data of Phase 3 trials for DURAVYU for wet AMD in 2026 –
–Company to host R&D Day in New York on June 26, 2024 –
WATERTOWN, Mass., May 8, 2024 (GLOBE NEWSWIRE) – EyePoint Pharmaceuticals, Inc. (NASDAQ: EYPT), a company committed to developing and commercializing therapeutics to improve the lives of patients with serious retinal diseases, today announced financial results for the first quarter ended March 31, 2024, and highlighted recent corporate developments.
“We have continued advancing our pipeline through significant milestones including the announcement of topline data from our Phase 2 PAVIA clinical trial of DURAVYU™ in non-proliferative diabetic retinopathy (NPDR),” said Jay Duker, M.D., President and Chief Executive Officer of EyePoint Pharmaceuticals. “Although the PAVIA trial did not meet the pre-specified primary endpoint, we were encouraged that DURAVYU demonstrated a biologic effect in patients with NPDR and continues to show a favorable safety and tolerability profile. As such, we plan to assess the full twelve-month study results once they are available to evaluate the path forward for DURAVYU as a potential treatment for NPDR. Looking ahead, we remain on track to initiate the first pivotal Phase 3 LUGANO non-inferiority clinical trial of DURAVYU in wet AMD in the second half of 2024 and for topline data for the Phase 2 VERONA trial in diabetic macular edema (DME) in the first quarter of 2025.”
R&D Highlights and Updates
•Announced topline efficacy and safety data from the Phase 2 PAVIA clinical trial of DURAVYU in NPDR in May. The data demonstrated that DURAVYU has a biologic effect in patients with NPDR with a favorable safety and tolerability profile; however, the trial did not meet the pre-specified primary endpoint. The Company plans to provide an update on the path forward for DURAVYU as a potential treatment in NPDR following a review of the full 12-month data in the third quarter of 2024.
•Completed an End-of-Phase 2 meeting with the U.S. Food and Drug Administration (FDA) in April to discuss pivotal Phase 3 clinical trial plans for DURAVYU in wet AMD. The Company will provide an update once final meeting minutes are received from the FDA.
•Presented the science and supporting clinical data for DURAVYU as a potentially disruptive innovation in the management of patients with wet AMD at the Ophthalmology Innovation Summit (OIS) Retina in May. Additionally, EyePoint was accepted to participate in a panel discussion at the Retina World Congress where the Company also plans to present an encore presentation of the DAVIO 2 clinical trial results.
•Presented four posters at the 2024 Association for Research in Vision and Ophthalmology (ARVO) Annual Meeting in May highlighting the durable efficacy, reliable safety, and reduced injection burden of treatment with DURAVYU. The topics presented include plasma PK data of single-and repeat-dose of DURAVYU, the mechanism of action of vorolanib and differentiation versus other
tyrosine kinase inhibitors, the design and function of DURAVYU as a sustained delivery platform for retinal disease, and an encore presentation of DAVIO 2 clinical trial results.
•Published Phase 1 DAVIO trial results in Ophthalmology Science in April, in article entitled, “Phase 1 DAVIO Trial: EYP-1901 Bioerodible, Sustained-Delivery Vorolanib Insert in Patients with Wet Age-related Macular Degeneration” (DOI: https://doi.org/10.1016/j.xops.2024.100527).
•Expanded the Company’s Scientific Advisory Board with world-renowned retina specialists including Usha Chakravarthy, M.B.B.S., Ph.D.; Allen Ho, M.D. FACS FASRS, and Frank Holz, M.D., F.E.B.O., F.A.R.V.O. to support advancement of the Company’s global clinical strategy for DURAVYU ahead of the anticipated initiation of its Phase 3 pivotal trials in wet AMD. Additionally, Charles Wykoff, M.D., Ph.D. joined Carl Regillo, M.D., FACS as co-chair of Scientific Advisory Board.
•EyePoint plans to host an R&D Day on June 26, 2024 in New York City. The event will feature commentary from management and KOL guest speakers. They will discuss the science behind EyePoint’s bioerodible Durasert E™ technology and the clinical and regulatory progress for their lead pipeline asset, DURAVYU, as well as an overview of the Company’s early pipeline programs. Additional details for the R&D Day to follow. KOL guest speakers include:
oYasha S. Modi, M.D., Associate Professor of Vitreoretinal Surgery, Retinal Disease and Uveitis at New York University; Director of Teleretina
oCarl Regillo M.D., FACS Professor of Ophthalmology at Thomas Jefferson University; Chief of Retina Service at Wills Eye Hospital; Founder of Wills Eye Clinical Retina Research Unit in Philadelphia
oRishi P. Singh M.D., Staff Physician, Vice-President and Chief Medical Officer of the Cleveland Clinical Martin Hospitals, Cleveland Clinic Florida, Stuart FL
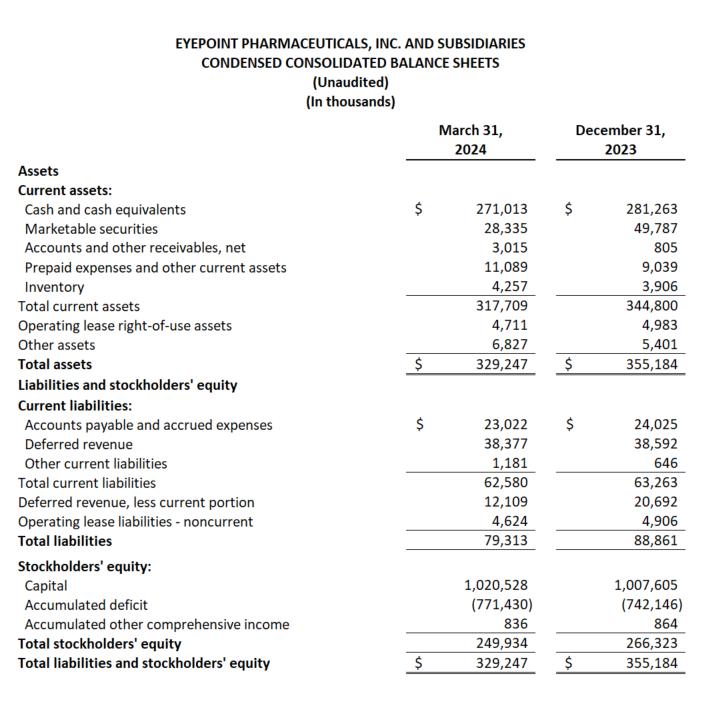
Review of Results for the First Quarter Ended March 31, 2024
For the first quarter ended March 31, 2024, total net revenue was $11.7 million compared to $7.7 million for the quarter ended March 31, 2023. Net product revenue for the first quarter was $0.7 million, compared to net product revenues for the first quarter ended March 31, 2023 of $7.4 million. This decrease in net product revenue resulted from the out-license of the YUTIQ franchise in May 2023, completing the strategic pivot from a commercial company to a biopharmaceutical pipeline-focused company.
Net revenue from royalties and collaborations for the first quarter ended March 31, 2024 totaled $11.0 million compared to $0.3 million in the corresponding period in 2023. This increase was primarily due to partial recognition of deferred revenue from the license of the YUTIQ franchise, which begun in 2Q 2023 and will be recognized over a 2-year period in connection with the delivery of YUTIQ supply units.
Operating expenses for the first quarter ended March 31, 2024 totaled $45.0 million versus $29.2 million in the prior year period. This increase was primarily driven by significant growth in research and development costs, including DURAVYU clinical trial activities and personnel expenses, and stock-based compensation offset by reduced sales and marketing expense from the exit of our commercial business in 1H 2023. Non-operating income, net, totaled $4.0 million and net loss was $29.3 million, or ($0.55) per share, compared to a net loss of $21.2 million, or ($0.56) per share, for the prior year period.
Cash and investments at March 31, 2024 totaled $299.3 million compared to $331.0 million at December 31, 2023.
Financial Outlook
We expect the cash, cash equivalents and investments on March 31, 2024, will enable us to fund operations through topline data for the planned Phase 3 clinical trials of DURAVYU for wet AMD in 2026.
DURAVYU™ has been conditionally accepted by the FDA as the proprietary name for EYP-1901. DURAVYU is an investigational product; it has not been approved by the FDA. FDA approval and the timeline for potential approval is uncertain.
About EyePoint Pharmaceuticals
EyePoint Pharmaceuticals (Nasdaq: EYPT) is a clinical-stage biopharmaceutical company committed to developing and commercializing therapeutics to help improve the lives of patients with serious retinal diseases. The Company's pipeline leverages its proprietary bioerodible Durasert E™ technology for sustained intraocular drug delivery. The Company’s lead product candidate, DURAVYU (previously known as EYP-1901), is an investigational sustained delivery treatment for VEGF-mediated retinal diseases combining vorolanib, a selective and patent-protected tyrosine kinase inhibitor with Durasert E™. Pipeline programs include EYP-2301, a promising TIE-2 agonist, razuprotafib, formulated in Durasert E™ to potentially improve outcomes in serious retinal diseases. The proven Durasert® drug delivery technology has been safely administered to thousands of patient eyes across four U.S. FDA approved products. EyePoint Pharmaceuticals is headquartered in Watertown, Massachusetts.
Vorolanib is licensed to EyePoint exclusively by Equinox Sciences, a Betta Pharmaceuticals affiliate, for the localized treatment of all ophthalmic diseases outside of China, Macao, Hong Kong and Taiwan.
Forward Looking Statements
EYEPOINT PHARMACEUTICALS SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION ACT OF 1995: To the extent any statements made in this press release deal with information that is not historical, these are forward-looking statements under the Private Securities Litigation Reform Act of 1995. Such statements include, but are not limited to, statements regarding the use of proceeds for the offering and other statements identified by words such as “will,” “potential,” “could,” “can,” “believe,” “intends,” “continue,” “plans,” “expects,” “anticipates,” “estimates,” “may,” other words of similar meaning or the use of future dates. Forward-looking statements by their nature address matters that are, to different degrees, uncertain. Uncertainties and risks may cause EyePoint’s actual results to be materially different than those expressed in or implied by EyePoint’s forward-looking statements. For EyePoint, this includes statements about the sufficiency of our existing cash resources through topline data for Phase 3 clinical trials for EYP-1901 (DURAVYU™) in wet AMD; our expectations regarding the timing and clinical development of our product candidates, including DURAVYU and EYP-2301; the potential for DURAVYU as a novel sustained delivery treatment for serious eye diseases, including wet age-related macular degeneration (wet AMD) and non-proliferative diabetic retinopathy (NPDR) and diabetic macular edema (DME); the effectiveness and timeliness of clinical trials, and the usefulness of the data; the timeliness of regulatory approvals including potential U.S. Food and Drug Administration (FDA) regulatory approval of DURAVYU and EYP-2301; the success of current and future license agreements; our dependence on contract research organizations, co-promotion partners, and other outside vendors and service providers; the success of Durasert® as a drug delivery platform in FDA approved products; product liability; industry consolidation; compliance with environmental laws; risks and costs of international business operations; volatility of stock price; possible

dilution; absence of dividends; the impact of general business and economic conditions; protection of our intellectual property and avoiding intellectual property infringement; retention of key personnel; manufacturing risks; and other factors described in our filings with the Securities and Exchange Commission. We cannot guarantee that the results and other expectations expressed, anticipated or implied in any forward-looking statement will be realized. A variety of factors, including these risks, could cause our actual results and other expectations to differ materially from the anticipated results or other expectations expressed, anticipated or implied in our forward-looking statements. Should known or unknown risks materialize, or should underlying assumptions prove inaccurate, actual results could differ materially from past results and those anticipated, estimated or projected in the forward-looking statements. You should bear this in mind as you consider any forward-looking statements. Our forward-looking statements speak only as of the dates on which they are made. EyePoint undertakes no obligation to update or revise any forward-looking statement, whether as a result of new information, future events, or otherwise.
Investors:
Christina Tartaglia
Stern IR
Direct: 212-698-8700
christina.tartaglia@sternir.com
Media Contact:
Amy Phillips
Green Room Communications
Direct: 412-327-9499
aphillips@greenroompr.com

v3.24.1.u1
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
EyePoint Pharmaceuticals (NASDAQ:EYPT)
Graphique Historique de l'Action
De Jan 2025 à Fév 2025

EyePoint Pharmaceuticals (NASDAQ:EYPT)
Graphique Historique de l'Action
De Fév 2024 à Fév 2025


