true
0001382574
S-1/A
1
1
1
1
P5Y
0001382574
2024-01-01
2024-06-30
0001382574
dei:BusinessContactMember
2024-01-01
2024-06-30
0001382574
SCNX:ScientureIncMember
2024-06-30
0001382574
SCNX:ScientureIncMember
2023-12-31
0001382574
SCNX:ScientureIncMember
2022-12-31
0001382574
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2021-12-31
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2021-12-31
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2021-12-31
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2021-12-31
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2021-12-31
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2022-12-31
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2022-12-31
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2022-12-31
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2022-12-31
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2022-12-31
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2023-06-30
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2023-06-30
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2023-06-30
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2023-06-30
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2023-06-30
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2023-12-31
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2024-06-30
0001382574
us-gaap:CommonStockMember
SCNX:ScientureIncMember
2024-06-30
0001382574
us-gaap:AdditionalPaidInCapitalMember
SCNX:ScientureIncMember
2024-06-30
0001382574
us-gaap:RetainedEarningsMember
SCNX:ScientureIncMember
2024-06-30
0001382574
SCNX:StockholdersDeficitMember
SCNX:ScientureIncMember
2024-06-30
0001382574
SCNX:ScientureIncMember
2023-06-30
0001382574
SCNX:ScientureIncMember
2021-12-31
0001382574
SCNX:ScientureIncMember
2024-03-01
2024-03-31
0001382574
SCNX:ScientureIncMember
2024-03-31
0001382574
us-gaap:PreferredStockMember
SCNX:ScientureIncMember
2024-03-01
2024-03-31
0001382574
SCNX:LoanAgreementMember
SCNX:ScientureIncMember
2023-09-30
0001382574
SCNX:LoanAgreementMember
SCNX:ScientureIncMember
2023-12-31
0001382574
SCNX:LoanAgreementMember
SCNX:ScientureIncMember
2024-06-30
0001382574
SCNX:LoanAgreementMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:LoanAgreementMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
SCNX:KesinPharmaCorporationMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
SCNX:KesinPharmaCorporationMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
SCNX:SaptalisPharmaceuticalsLlcMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
SCNX:SaptalisPharmaceuticalsLlcMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
srt:DirectorMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
SCNX:PromissoryNoteMember
us-gaap:SubsequentEventMember
SCNX:ScientureIncMember
2024-07-31
0001382574
SCNX:KesinPharmaCorporationMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:KesinPharmaCorporationMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:SaptalisPharmaceuticalsLlcMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:SaptalisPharmaceuticalsLlcMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2024-06-30
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2022-12-31
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:ScientureIncMember
us-gaap:GeneralAndAdministrativeExpenseMember
2024-01-01
2024-06-30
0001382574
SCNX:ScientureIncMember
us-gaap:GeneralAndAdministrativeExpenseMember
2023-01-01
2023-06-30
0001382574
SCNX:ScientureIncMember
us-gaap:GeneralAndAdministrativeExpenseMember
2023-01-01
2023-12-31
0001382574
SCNX:ScientureIncMember
us-gaap:GeneralAndAdministrativeExpenseMember
2022-01-01
2022-12-31
0001382574
srt:MinimumMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
srt:MaximumMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
srt:MinimumMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
srt:MaximumMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
srt:MinimumMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
srt:MaximumMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
srt:MinimumMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
srt:MaximumMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
us-gaap:WarrantMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
us-gaap:WarrantMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
SCNX:ConvertiblePreferredStockAndConvertibleNotesMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
SCNX:ConvertiblePreferredStockAndConvertibleNotesMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
SCNX:LongTermConvertibleDebtMember
SCNX:ScientureIncMember
2024-01-01
2024-06-30
0001382574
SCNX:LongTermConvertibleDebtMember
SCNX:ScientureIncMember
2023-01-01
2023-06-30
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
us-gaap:EmployeeStockOptionMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:WarrantMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
us-gaap:WarrantMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:ConvertiblePreferredStockAndConvertibleNotesMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:ConvertiblePreferredStockAndConvertibleNotesMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:LongTermConvertibleDebtMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:LongTermConvertibleDebtMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
us-gaap:SubsequentEventMember
SCNX:ScientureIncMember
2024-07-31
0001382574
us-gaap:SubsequentEventMember
SCNX:ScientureIncMember
2024-03-01
2024-03-31
0001382574
us-gaap:SubsequentEventMember
SCNX:ScientureIncMember
2024-03-31
0001382574
SCNX:MsPushpaShankarMember
SCNX:IssueOfPreferredStockMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:MsPushpaShankarMember
SCNX:IssueOfPreferredStockMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:MsPushpaShankarMember
SCNX:IssueOfPreferredStockMember
SCNX:ScientureIncMember
2022-12-31
0001382574
SCNX:MsPushpaShankarMember
SCNX:IssueOfPreferredStockMember
SCNX:ScientureIncMember
2023-12-31
0001382574
SCNX:MsPushpaShankarMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:MsPushpaShankarMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:MsPushpaShankarMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2022-12-31
0001382574
SCNX:MsPushpaShankarMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2023-12-31
0001382574
SCNX:MsYogitaDesaiMember
SCNX:IssueOfPreferredStockMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:MsYogitaDesaiMember
SCNX:IssueOfPreferredStockMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:MsYogitaDesaiMember
SCNX:IssueOfPreferredStockMember
SCNX:ScientureIncMember
2022-12-31
0001382574
SCNX:MsYogitaDesaiMember
SCNX:IssueOfPreferredStockMember
SCNX:ScientureIncMember
2023-12-31
0001382574
SCNX:MsYogitaDesaiMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:MsYogitaDesaiMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:MsYogitaDesaiMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2022-12-31
0001382574
SCNX:MsYogitaDesaiMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2023-12-31
0001382574
SCNX:MrSandeepGuptaMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2023-01-01
2023-12-31
0001382574
SCNX:MrSandeepGuptaMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2022-01-01
2022-12-31
0001382574
SCNX:MrSandeepGuptaMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2022-12-31
0001382574
SCNX:MrSandeepGuptaMember
SCNX:IssueOfConvertibleNotesMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:MeasurementInputExpectedDividendRateMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:MeasurementInputExpectedDividendRateMember
SCNX:ScientureIncMember
2022-12-31
0001382574
us-gaap:MeasurementInputPriceVolatilityMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:MeasurementInputPriceVolatilityMember
SCNX:ScientureIncMember
2022-12-31
0001382574
us-gaap:MeasurementInputRiskFreeInterestRateMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:MeasurementInputRiskFreeInterestRateMember
SCNX:ScientureIncMember
2022-12-31
0001382574
us-gaap:MeasurementInputExpectedTermMember
SCNX:ScientureIncMember
2023-12-31
0001382574
us-gaap:MeasurementInputExpectedTermMember
SCNX:ScientureIncMember
2022-12-31
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
xbrli:pure
utr:MBbls
SCNX:Integer
As
filed with the Securities and Exchange Commission on January 13, 2025
Registration
No. 333-283591
UNITED STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington, D.C. 20549
Amendment No. 1
to
FORM S-1
REGISTRATION STATEMENT
UNDER
THE SECURITIES ACT OF 1933
Scienture Holdings, Inc.
(Exact name of Registrant as specified in its charter)
| Delaware |
|
5122 |
|
46-3673928 |
(State
or other jurisdiction of
incorporation
or organization) |
|
(Primary
Standard Industrial
Classification
Code Number) |
|
(I.R.S.
Employer
Identification
No.) |
6308 Benjamin Rd, Suite 708
Tampa, Florida 33634
(800) 261-0281
(Address,
including zip code and telephone number, including area code, of Registrant’s principal executive offices)
Surendra Ajjarapu
Chief Executive Officer
Scienture Holdings, Inc.
6308 Benjamin Rd, Suite 708
Tampa, Florida 33634
(800) 261-0281
(Name,
address, including zip code, and telephone number, including area code, of agent for service)
Copies to:
Kate
L. Bechen
Louis D. Kern
Dykema Gossett PLLC
111 E Kilbourn Ave, Suite 1050
Milwaukee, Wisconsin 53202
(414) 488-7300
Approximate
date of commencement of proposed sale to the public: As soon as practicable after the effective date of this registration statement.
If
any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the
Securities Act of 1933 check the following box. ☒
If
this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, check the following
box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering.
☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If
this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the
Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate
by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting
company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,”
“smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
| Large
accelerated filer |
☐ |
Accelerated
filer |
☐ |
| Non-accelerated
filer |
☒ |
Smaller
reporting company |
☒ |
| |
|
Emerging
growth company |
☐ |
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 7(a)(2)(B) of the Securities Act. ☐
The
Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the
Registrant shall file a further amendment which specifically states that this Registration Statement shall thereafter become effective
in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective
on such date as the Securities and Exchange Commission, acting pursuant to said Section 8(a), may determine.
The
information in this preliminary prospectus is not complete and may be changed. We may not sell these securities until the registration
statement filed with the Securities and Exchange Commission is effective. This preliminary prospectus is not an offer to sell these securities
and it is not soliciting offers to buy these securities in any state or other jurisdiction where the offer or sale is not permitted.
PRELIMINARY
PROSPECTUS SUBJECT TO COMPLETION, DATED JANUARY 13, 2025

SCIENTURE HOLDINGS, INC.
4,300,000 SHARES
OF COMMON STOCK
This
prospectus relates to the resale from time to time of up to 4,300,000 shares of common stock, par value $0.00001, of Scienture
Holdings, Inc. (f/k/a TRxADE HEALTH, INC.), a Delaware corporation (“we,” “us,” “our,” or the “Company”),
by Arena Finance Markets, LP (“Arena Finance”), Arena Special Opportunities Partners III, LP (“ASOP” and,
together with Arena Finance, the “Arena Investors”), and Arena Business Solutions Global SPC II, Ltd (“Arena Global”
and, together with the Arena Investors, the “Selling Stockholders”).
We
are registering the resale of up to 4,300,000 shares of common stock, comprised of (i) 3,925,000 ELOC Shares (as
defined below), (ii) 70,000 shares of common stock (the “Initial Commitment Fee Shares”) issued to Arena Global as a
commitment fee upon the execution of a purchase agreement dated November 25, 2024 (the “ELOC Purchase
Agreement”), (iii) up to 250,000 shares of common stock issuable to Arena Global as an additional commitment fee
pursuant to the ELOC Purchase Agreement (the “Additional Commitment Fee Shares” and, together with the Initial
Commitment Fee Shares, the “ELOC Commitment Fee Shares”), and (iv) 55,000 shares of common stock (the
“SPA Commitment Fee Shares”) issued to the Arena Investors as a commitment fee upon the execution of a securities
purchase agreement dated November 22, 2024 (the “Securities Purchase Agreement”). See “The Arena Global Transactions”
for a description of the terms and conditions of the ELOC Purchase Agreement and the Securities Purchase Agreement, including
the ELOC Shares, the ELOC Commitment Fee Shares, and the SPA Commitment Fee Shares.
The
prices at which the Selling Stockholders may resell the shares offered hereby will be determined by the prevailing market price
for the shares or in negotiated transactions. We are not selling any securities under this prospectus and will not receive any of the
proceeds from the sale of shares of common stock by the Selling Stockholders. However, we may receive up to $50,000,000 in
aggregate gross proceeds under the ELOC Purchase Agreement and we have received approximately $3,000,000 of gross proceeds in connection
with the first closing under the Securities Purchase Agreement.
The
Selling Stockholders may sell the shares of common stock described in this prospectus in a number of different ways and at varying
prices. We provide more information about how the Selling Stockholders may sell their shares of common stock in the section
titled “Plan of Distribution” on page 45 of this prospectus.
Each
Selling Stockholder is an “underwriter”
within the meaning of Section 2(a)(11) of the Securities Act of 1933, as amended (the “Securities Act”) with respect to the
resale of their shares of common stock hereunder.
We
will pay the expenses incurred in registering the securities covered by this prospectus, including legal and accounting fees. To the
extent the Selling Stockholders decide to sell their shares of common stock we will not control or determine the price
at which the shares are sold.
You
should read this prospectus, together with additional information described under the heading “Where You Can Find More Information”
carefully before you invest in any of our securities.
Our
common stock is listed on the Nasdaq Stock Market LLC (“Nasdaq”) under the symbol “SCNX”. The last reported sale
price of our common stock on Nasdaq on January 8, 2025 was $5.07 per share. We urge prospective purchasers
of our common stock to obtain current information about the market prices of the common stock.
INVESTING
IN OUR SECURITIES INVOLVES RISKS. YOU SHOULD REVIEW CAREFULLY THE RISKS AND UNCERTAINTIES DESCRIBED UNDER THE HEADING “RISK
FACTORS” BEGINNING ON PAGE 8 HEREIN, AS WELL AS OUR PERIODIC AND CURRENT REPORTS WHICH WE HAVE FILED WITH THE SECURITIES
AND EXCHANGE COMMISSION, WHICH ARE INCORPORATED BY REFERENCE INTO THIS PROSPECTUS. YOU SHOULD READ THE ENTIRE PROSPECTUS CAREFULLY BEFORE
YOU MAKE YOUR INVESTMENT DECISION.
NEITHER
THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED
UPON THE ACCURACY OR ADEQUACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The
date of this Prospectus is [●], 2024.
TABLE
OF CONTENTS
ABOUT
THIS PROSPECTUS
Scienture
Holdings, Inc. and its consolidated subsidiaries are referred to herein as “Scienture,” “the Company,”
“we,” “us” and “our,” unless the context indicates otherwise.
This
prospectus is part of a registration statement on Form S-1 that we filed with the U.S. Securities and Exchange Commission (the “SEC”)
pursuant to which the Selling Stockholders may, from time to time, offer and sell or otherwise dispose of the shares of our common
stock covered by this prospectus. We will not receive any proceeds from the sale by the Selling Stockholders of the shares
offered by them described in this prospectus. We may also file a prospectus supplement or post-effective amendment to the
registration statement of which this prospectus forms a part that may contain material information relating to this offering. The prospectus
supplement or post-effective amendment may also add, update or change information contained in this prospectus. If there is any inconsistency
between the information in this prospectus and the applicable prospectus supplement or post-effective amendment, you should rely on the
prospectus supplement or post-effective amendment, as applicable. The registration statement we filed with the SEC, of which this prospectus
forms a part, includes exhibits that provide more detail of the matters discussed in this prospectus. You should read this prospectus,
any post-effective amendment, and any applicable prospectus supplement and the related exhibits filed with the SEC before making your
investment decision. The registration statement and the exhibits can be obtained from the SEC, as indicated under the section entitled
“Where You Can Find More Information.”
We
incorporate by reference important information into this prospectus. You may obtain the information incorporated by reference without
charge by following the instructions under “Where You Can Find More Information.” You should carefully read this prospectus
as well as additional information described under “Information Incorporated By Reference” before deciding to invest
in our securities.
You
should rely only on the information contained in this prospectus. Neither we nor the Selling Stockholders have authorized anyone
to provide you with any information or to make any representations other than those contained in this prospectus, any post-effective
amendment, or any applicable prospectus supplement prepared by or on behalf of us or to which we have referred you. We and the Selling
Stockholders take no responsibility for and can provide no assurance as to the reliability of any other information that others
may give you. If anyone provides you with different or inconsistent information, you should not rely on it. You should assume that the
information appearing in this prospectus, any post-effective amendment and any applicable prospectus supplement to this prospectus is
accurate only as of the date on its respective cover. Our business, financial condition, results of operations and prospects may have
changed since those dates. You should read carefully the entirety of this prospectus before making an investment decision.
Neither
we nor the Selling Stockholders are making an offer to sell our common stock in any jurisdiction where the offer
or sale thereof is not permitted. The distribution of this prospectus and the offering of our common stock in certain jurisdictions may
be restricted by law. Persons outside the United States who come into possession of this prospectus must inform themselves about, and
observe any restrictions relating to, the offering of our common stock and the distribution of this prospectus outside the United States.
This prospectus does not constitute, and may not be used in connection with, an offer to sell, or a solicitation of an offer to buy,
any securities offered by this prospectus by any person in any jurisdiction in which it is unlawful for such person to make such an offer
or solicitation.
This
prospectus contains summaries of certain provisions contained in some of the documents described herein, but reference is made to the
actual documents for complete information. All of the summaries are qualified in their entirety by the actual documents. Copies of some
of the documents referred to herein have been filed, will be filed or will be incorporated by reference as exhibits to the registration
statement of which this prospectus is a part, and you may obtain copies of those documents as described below under the section entitled
“Where You Can Find More Information.”
PROSPECTUS SUMMARY
This
summary highlights information contained elsewhere in this prospectus or incorporated by reference herein and does not contain all the
information that you should consider in making your investment decision. Before investing in our common stock, you should read the entire
prospectus carefully, including the risks of investing in our securities discussed under the heading “Risk Factors” contained
in this prospectus and under similar headings in the other documents that are incorporated by reference into this prospectus. Prospective
purchasers of our securities should also carefully read the information incorporated by reference into this prospectus, including our
financial statements, and the exhibits to the registration statement of which this prospectus is a part.
Company
Overview
We
historically focused on health services IT assets and operations aimed at digitalizing the retail pharmacy experience via an online pharmaceutical
marketplace. Our legacy operations are currently conducted through Integra Pharma Solutions, LLC (“IPS”), which is a licensed
pharmaceutical wholesaler and sells brand, generic and non-drug products to customers. IPS’ customers span various healthcare markets
including government organizations, hospitals, clinics and independent pharmacies nationwide.
On
July 25, 2024, we acquired a wholly-owned subsidiary, Scienture, LLC (f/k/a Scienture, Inc.) (“Scienture LLC”), which
is a specialty pharmaceutical company focused on developing and commercializing products for the treatment of central nervous system
and cardiovascular diseases. Scienture LLC is developing a broad range of novel product candidates including new potential treatments
for hypertension, migraine, pain and thrombosis and other related disorders. The intellectual property application process was initiated
in November 2019 and the product development activities commenced in January 2020. Scienture LLC’s assets in development
are across therapeutics areas and indications and cater to different market segments. Scienture LLC’s mission is to identify,
develop and bring to market innovative technology-based products to address unmet medical needs. Its targeted portfolio consists of short
term and long-term opportunities with efficient development, regulatory, and go to market strategies.
On
September 20, 2024, we changed the legal name of the Company from “TRxADE HEALTH, Inc.” to “Scienture Holdings, Inc.”
As
of September 30, 2024, we owned 100% of Softell Inc. (f/k/a Trxade Inc.), IPS, Bonum Health, LLC, Bonum Health Inc., and Scienture LLC.
On
October 4, 2024, we entered into an Assignment and Assumption of Membership Interests (the “IPS Assignment Agreement”) with
our wholly-owned subsidiary, Softell Inc. (f/k/a Trxade Inc.) (“Softell”), pursuant to which we transferred, and Softell
accepted, 100% of the membership interests of IPS. As a result, IPS became a wholly-owned subsidiary of Softell and Softell’s current
primary operations are conducted through IPS.
Bonum
Health, LLC was formed to hold certain telehealth assets acquired in October 2019. The “Bonum Health Hub” was launched in
February 2020. However, we do not anticipate installations moving forward and we anticipate dissolving Bonum Health, Inc. and Bonum Health,
LLC.
Corporate
Information
Our
principal business address is 6308 Benjamin Rd, Suite 708, Tampa, Florida 33634 and our telephone number is (800) 261-0281. We maintain
our corporate website at https://scienture.com. The reference to our website is intended to be an inactive textual reference only.
Information on our website does not constitute a part of, nor is it incorporated in any way, into this prospectus and should not be relied
upon in connection with making an investment decision. Our common stock is listed on Nasdaq under the symbol “SCNX”.
Status
as a Public Company
We are a “smaller
reporting company” as defined in Item 10(f)(1) of Regulation S-K. Smaller reporting companies may take advantage of certain reduced
disclosure obligations, including, among other things, providing only two years of audited financial statements. We will remain a smaller
reporting company until the last day of the fiscal year in which (1) the market value of our shares of common stock held by non-affiliates
exceeds $250 million as of the last business day of that year’s second fiscal quarter, or (2) our annual revenues exceeded $100
million during such completed fiscal year and the market value of our shares of common stock held by non-affiliates equals or exceeds
$700 million as of the last business day of that year’s second fiscal quarter.
The
Arena Global Transactions
ELOC
Purchase Agreement
On
November 25, 2024, we entered into the ELOC Purchase Agreement with Arena Global, pursuant to which we have the right, but not the obligation,
to direct Arena Global to purchase up to $50,000,000 in shares of our common stock (the “ELOC Shares”) upon satisfaction
of certain terms and conditions contained in the ELOC Purchase Agreement, which includes, but is not limited to, filing a registration
statement with the SEC and registering the resale of any shares sold to Arena Global. The term of the ELOC Purchase Agreement began on
the date of execution and ends on the earlier of (i) December 1, 2027, (ii) the date on which Arena Global shall have purchased the maximum
amount of ELOC Shares, or (iii) the effective date of any written notice of termination delivered pursuant to the terms of the ELOC Purchase
Agreement (the “Commitment Period”).
During
the Commitment Period, the Company may direct Arena Global to purchase ELOC Shares by delivering a notice (an “Advance Notice”)
to Arena Global. The Company shall, in its sole discretion, select the amount of ELOC Shares requested by the Company in each Advance
Notice. However, such amount may not exceed the Maximum Advance Amount, which is calculated as follows:
| (a) | if
the Advance Notice is received by 8:30 a.m. Eastern Time, the lower of: (i) an amount equal
to eighty percent (80%) of the average of the Daily Value Traded (as defined in the ELOC
Purchase Agreement) of our common stock on the ten (10) trading days immediately preceding
an Advance Notice, or (ii) $20,000,000; |
| (b) | if
the Advance Notice is received after 8:30 a.m. Eastern Time but prior to 10:30 a.m. Eastern
Time, the lower of: (i) an amount equal to forty percent (40%) of the average of the Daily
Value Traded of our common stock on the ten (10) trading days immediately preceding an Advance
Notice, or (ii) $10,000,000; |
| | | |
| (c) | if
the Advance Notice is received after 10:30 a.m. Eastern Time, but prior to 12:30 p.m. Eastern
Time, the lower of: (i) an amount equal to twenty percent (20%) of the average of the Daily
Value Traded of our common stock on the ten (10) trading days immediately preceding an Advance
Notice, or (ii) $5,000,000; and |
| | | |
| (d) | if
the Advance Notice is received after 12:30 p.m. Eastern Time but prior to 2:30 p.m. Eastern
Time, the lower of: (i) an amount equal to ten percent (10%) of the average of the Daily
Value Traded of our common stock on the ten (10) trading days immediately preceding an Advance
Notice, or (ii) $2,500,000. |
The
purchase price to be paid by Arena Global for the ELOC Shares will be ninety-six percent (96%) of the VWAP (as defined in the ELOC Purchase
Agreement) of the Company’s common stock during the trading day commencing on the date of the Advance Notice, subject to adjustment
pursuant to the terms of the ELOC Purchase Agreement.
At
any given time of any sale by us to Arena Global, we may not sell, and Arena Global may not purchase, ELOC Shares that would result in
Arena Global owning more than 9.99% of our outstanding common stock upon such issuance (the “Beneficial Ownership Limitation”).
Additionally, the Company must obtain shareholder approval to issue an aggregate number of shares of common stock to Arena Global, under
the ELOC Purchase Agreement, in excess of 19.99% of the number of shares of common stock outstanding immediately prior to the execution
of the ELOC Purchase Agreement prior to delivering any Advance Notices. For purposes of the foregoing, and the Beneficial Ownership Limitation,
any additional shares issued pursuant to the terms of the Securities Purchase Agreement described below will be aggregated with
the ELOC Shares, ELOC Commitment Fee Shares, and SPA Commitment Fee Shares.
In
consideration for Arena Global’s execution and delivery of the ELOC Purchase Agreement, we agreed to issue to Arena Global, as
a commitment fee: (i) 70,000 Initial Commitment Fee Shares and (ii) in two separate tranches, a number of Additional Commitment Fee Shares
equal to (a) with respect to the first tranche, 500,000 divided by the simple average of the daily VWAP of our common stock during the
five (5) trading days immediately preceding the effectiveness (the “Effectiveness Date”) of the initial registration statement
on which the ELOC Commitment Fee Shares are registered (the “First Trance Price”) and (b) with respect to the second
tranche, 500,000 divided by the simple average of the daily VWAP of our common stock during the five (5) trading days immediately preceding
the two (2) month anniversary (the “Anniversary”) of the Effectiveness Date (the “Second Tranche Price”). The
Additional Commitment Fee Shares shall be subject to a true-up after each issuance pursuant to the terms of the ELOC Purchase Agreement.
We are registering up to 320,000 ELOC Commitment Fee Shares hereunder.
Under
the ELOC Purchase Agreement we also agreed to, no later than ten (10) business days following the execution date, file with the SEC a
registration statement for the resale by Arena Global of the ELOC Shares and the ELOC Commitment Fee Shares, and to file one or
more additional registration statements if necessary. This registration statement on Form S-1 (“Registration Statement”)
is being filed in order to satisfy our obligations under the ELOC Purchase Agreement related to registering for resale the ELOC Shares
and the ELOC Commitment Fee Shares.
The
ELOC Purchase Agreement contains customary representations, warranties, agreements and conditions to completing future sale transactions,
indemnification rights and obligations of the parties. Among other things, Arena Global represented to us, that it is an “accredited
investor” (as such term is defined in Rule 501(a) of Regulation D under the Securities Act). We will sell the securities in reliance
upon an exemption from registration contained in Section 4(a)(2) of the Securities Act and Regulation D promulgated thereunder.
Securities
Purchase Agreement
On
November 22, 2024, the Company entered into the Securities Purchase Agreement with
the Arena Investors. Under the Securities Purchase Agreement, the Company will issue 10%
original issue discount secured convertible debentures (“Debentures”) in a principal amount of up to $12,222,222 million,
divided into up to three separate tranches that are each subject to certain closing conditions. The conversion price per share of each
Debenture is equal to 92.5% of the lowest daily VWAP (as defined in the Debentures) of the Company’s shares of common stock during
the five trading day period ending on the trading day immediately prior to delivery or deemed delivery of the applicable Conversion Notice
(as defined in the Debentures), subject to adjustments related to the trading price of the Company’s common stock.
The
closing of the first tranche was consummated on November 25, 2024 (the “First Closing”) and the Company issued to the Arena
Investors Debentures in an aggregate principal amount of $3,333,333 (the “First Closing Debentures”). The First Closing Debentures
were sold to the Arena Investors for a purchase price of $3,000,000, representing an original issue discount of ten percent (10%).
The
First Closing Debentures contain customary events of default. If an event of default occurs, until it is cured, the holder may increase
the interest rate applicable to the First Closing Debentures to two percent (2%) per annum and accelerate the full indebtedness under
the First Closing Debentures, in an amount equal to 125% of the outstanding principal amount and accrued and unpaid interest. Subject
to limited exceptions set forth in the First Closing Debentures, the First Closing Debentures prohibit the Company and, as applicable,
its subsidiaries from incurring any new indebtedness that is not subordinated to the Arena Investors and, as applicable, any subsidiary’s
obligations in respect of the First Closing Debentures until the First Closing Debentures are paid in full.
As
consideration for the Arena Investors’ consummation of the First Closing, concurrently with the First Closing, the Company issued
to each Arena Investor participating in the First Closing its pro rata portion of 55,000 shares of Company common stock. Furthermore,
as consideration for the Arena Investors’ consummation of subsequent closings, the Company shall issue to the Arena Investors participating
in such closing a certain number of Company common stock as agreed upon among the Company and the Arena Investors participating.
The
Company agreed, pursuant to a Security Agreement, dated November 25, 2024 (the “Security Agreement”), with the Arena Investors,
to grant the Arena Investors a security interest in all of its assets to secure the prompt payment, performance, and discharge in full
of all of the Company’s obligations under the Debentures. In addition, the Company’s wholly-owned subsidiary, Scienture
LLC, entered into a Guarantee Agreement, dated November 25, 2024 (the “Guarantee”), with the Arena Investors, pursuant to
which it agreed to guarantee the prompt payment, performance, and discharge in full of all of the Company’s obligations under the
Debentures.
The
Company also agreed, pursuant to a Registration Rights Agreement, dated November 25, 2024 (the “Registration Rights Agreement”),
with the Arena Investors to file with the SEC an initial registration statement within 45 days to register the maximum number of Registrable
Securities (as defined in the Registration Rights Agreement) in accordance with applicable SEC rules.
The
Securities Purchase Agreement, Debentures, Security Agreement, Guaranty Agreement, and Registration Rights Agreement contain customary
representations, warranties, agreements and conditions to completing future sale transactions, indemnification rights and obligations
of the parties. Among other things, the Arena Investors represented to the Company, that they are each an “accredited investor”
(as such term is defined in Rule 501(a) of Regulation D under the Securities Act). The Company issued, and will issue, the securities
in reliance upon an exemption from registration contained in Section 4(a)(2) of the Securities Act and Regulation D promulgated thereunder.
THE
OFFERING
| Common
Stock Offered by the Selling Stockholders |
|
Up
to 4,300,000 shares of common stock. |
| |
|
|
| Common
Stock Outstanding Prior to this Offering |
|
8,750,582
shares of common stock (as of January 3,
2025, and including (i) the 70,000 Initial Commitment Fee Shares, which have been issued to Arena Global, and (ii) the 55,000
SPA Commitment Fee Shares, which have been issued to the Arena Investors). |
| |
|
|
| Common
Stock Outstanding After this Offering |
|
12,925,582
shares of common stock, assuming the sale of
all of the ELOC Shares and the issuance of 250,000 Additional Commitment Fee Shares. |
| |
|
|
| Terms
of the Offering |
|
The
Selling Stockholders and any of their pledgees, assignees and successors-in-interest will determine when and how they
sell the shares offered in this prospectus and may, from time to time, sell any or all of their shares covered hereby on Nasdaq or
any other stock exchange, market or trading facility on which the shares are traded or in privately negotiated transactions. These
sales may be at fixed or negotiated prices. For more information, see “Plan of Distribution.” |
| |
|
|
| Use
of Proceeds |
|
The
Selling Stockholders will receive all of the proceeds from the sale of the shares
offered for sale by them under this prospectus. We will not receive proceeds from
the sale of the shares by the Selling Stockholders.
We
may receive up to $50,000,000 in aggregate gross proceeds under the ELOC Purchase Agreement in connection with sales of our shares
of common stock to Arena Global that we may, in our discretion, elect to make, from time to time pursuant to the ELOC Purchase
Agreement after the date of this prospectus. We intend to use the proceeds from the sale of our shares of common stock to Arena
Global for general corporate and working capital purposes. Our management will have broad discretion over the use of proceeds
from the sale of our shares of common stock under the ELOC Purchase Agreement.
For
more information, see “Use of Proceeds.” |
| |
|
|
| Risk
Factors |
|
Investing
in our securities involves a high degree of risk. You should carefully read the section titled “Risk Factors”
beginning on page 8 and the other information included in this prospectus for a discussion of factors you should consider
carefully before deciding to invest in our common stock. |
| |
|
|
| Market
for the Common Stock |
|
Our
common stock is listed on Nasdaq under the symbol “SCNX”. |
The
number of shares of our common stock that are and will be outstanding immediately before and after this offering as shown above is based
on 8,750,582 shares of common stock outstanding as of January 3, 2025 and excludes:
| ● | 190,242
shares
of common stock issuable upon exercise of warrants (as of January 3, 2025); |
| | | |
| ● | 1,575,900
shares
of common stock issuable upon conversion of preferred stock (as January 3, 2025);
and |
| | | |
| ● | 23,930
shares of common stock issuable upon exercise of
options (as of January 3, 2025). |
CAUTIONARY
NOTE REGARDING FORWARD-LOOKING STATEMENTS
This
Registration Statement contains statements that constitute forward-looking statements which are subject to the safe-harbor provisions
of the Private Securities Litigation Reform Act of 1995. Statements that are not historical are forward-looking statements within the
meaning of Section 27A of the Securities Act and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange
Act”). Some of the statements in this Registration Statement constitute forward-looking statements because they relate to future
events or our future performance or future financial condition. These forward-looking statements are not historical facts, but rather
are based on current expectations, estimates and projections about our company, our industry, our beliefs and our assumptions. Our forward-looking
statements include, but are not limited to, statements regarding our or our management team’s expectations, hopes, beliefs, intentions
or strategies regarding the future. In addition, any statements that refer to projections, forecasts or other characterizations of future
events or circumstances, including any underlying assumptions, are forward-looking statements. In some cases, the words “anticipate,”
“believe,” “continue,” “could,” “estimate,” “expect,” “intend,”
“may,” “ongoing,” “plan,” “potential,” “predict,” “project,”
“should,” or the negative of these terms or other similar expressions, may identify forward-looking statements, but the absence
of these words does not mean that a statement is not forward-looking. These forward-looking statements are subject to known and unknown
risks, uncertainties and assumptions about the Company that may cause the Company’s actual results, levels of activity, performance
or achievements to be materially different from any future results, levels of activity, performance or achievements expressed or implied
by such forward-looking statements.
Actual
performance or results could differ materially from those expressed in or suggested by the forward-looking statements. Important factors
that could cause such differences include, but are not limited to:
| |
● |
risks
related to our history of operating losses and that our operations may not become profitable; |
| |
|
|
| |
● |
claims
relating to alleged violations of intellectual property rights of others; |
| |
|
|
| |
● |
our
ability to manage our growth; |
| |
|
|
| |
● |
regulatory
and licensing requirement risks; |
| |
|
|
| |
● |
risks
related to changes in the U.S. healthcare environment; |
| |
|
|
| |
● |
risks
associated with the operations of our more established competitors; |
| |
|
|
| |
● |
our
ability to respond to general economic conditions, including financial market volatility and disruption, elevated levels of inflation,
and declining economic conditions in the United States; |
| |
|
|
| |
● |
changes
in laws or regulations relating to our operations; |
| |
|
|
| |
● |
our
growth strategy; |
| |
|
|
| |
● |
the
actual number of shares we will issue or sell under the ELOC Purchase Agreement to Arena Global; |
| |
|
|
| |
● |
compliance
with the continued listing requirements of Nasdaq; |
| |
|
|
| |
● |
risks
relating to the liquidity and trading of our common stock; |
| |
|
|
| |
● |
our
ability to raise financing in the future; |
| |
|
|
| |
● |
demand
for our products and services may decline; |
| |
|
|
| |
● |
data
security breaches, cyber-attacks or other network outages; and |
| |
|
|
| |
● |
other
risks disclosed below under, and incorporated by reference in, “Risk Factors.” |
The
forward-looking statements contained in this Registration Statement are based on our current expectations and beliefs concerning future
developments and their potential effects on us. There can be no assurance that future developments affecting us will be those that we
have anticipated. These forward-looking statements involve a number of risks, uncertainties (some of which are beyond our control) or
other assumptions that may cause actual results or performance to be materially different from those expressed or implied by these forward-looking
statements. These risks and uncertainties include, but are not limited to, those factors described under the section titled “Risk
Factors.” Should one or more of these risks or uncertainties materialize, or should any of our assumptions prove incorrect,
actual results may vary in material respects from those projected in these forward-looking statements.
Although
we believe that the assumptions on which these forward-looking statements are based are reasonable, any of those assumptions could prove
to be inaccurate, and as a result, the forward-looking statements based on those assumptions also could be inaccurate. In light of these
and other uncertainties, the inclusion of a projection or forward-looking statements in this Registration Statement should not be regarded
as a representation by us that our plans and objectives will be achieved.
We
have based the forward-looking statements included in this Registration Statement on information available to us on the date of this
Registration Statement, and we assume no obligation to update any such forward-looking statements. Although we undertake no obligation
to revise or update any forward-looking statements in this Registration Statement, whether as a result of new information, future events
or otherwise, you are advised to consult any additional disclosures that we may make directly to you or through reports that we may file
in the future with the SEC, including Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K.
RISK
FACTORS
Investing
in our securities involves a high degree of risk. Please see the risk factors under the heading “Risk Factors” in our Annual
Report on Form 10-K for the year ended December 31, 2023, on file with the SEC, and those risk factors identified in reports subsequently
filed with the SEC, including our Quarterly Reports on Form 10-Q, which are incorporated by reference into this prospectus. Before you
invest in our securities, you should carefully consider these risks as well as other information we include or incorporate by reference
into this prospectus. All of these risk factors are incorporated herein in their entirety. The risks and uncertainties we have described
are not the only ones facing our company. Additional risks and uncertainties not presently known to us or that we currently deem immaterial
may also affect our business operations. The occurrence of any of these risks might cause you to lose all or part of your investment
in the offered securities. Certain statements in this section, or which are incorporated by reference in this section, are forward-looking
statements. For more information, see “Cautionary Note Regarding Forward-Looking Statements” and “Where You Can Find
More Information.”
Risks
Related to this Offering and Our Common Stock
It
is not possible to predict the actual number of shares we will issue to Arena Global.
The
purchase price of the ELOC Shares will be ninety six percent (96%) of the VWAP (as defined in the ELOC Purchase Agreement) of the Company’s
common stock during the trading day commencing on the date of the Advance Notice, subject to adjustment pursuant to the terms of the
ELOC Purchase Agreement. Accordingly, the number of ELOC Shares issuable pursuant to the ELOC Purchase Agreement cannot be determined
at this time and may change over time.
Investors
who buy shares at different times will likely pay different prices.
Investors
who purchase shares in this offering at different times will likely pay different prices, and so may experience different levels of dilution
and different outcomes in their investment results. The Selling Stockholders may sell such shares at different times and at different
prices. Investors may experience a decline in the value of the shares they purchase from the Selling Stockholders in this offering
as a result of sales made by us in future transactions to the Selling Stockholders at prices lower than the prices they paid.
The
issuance of common stock to the Selling Stockholders may cause substantial dilution to our existing stockholders and the sale
of such shares acquired by the Selling Stockholders could cause the price of our common stock to decline.
We
are registering for resale by the Selling Stockholders up to 4,300,000 shares of common stock, comprised of (i) 3,925,000
ELOC Shares, (ii) 70,000 Initial Commitment Fee Shares, (iii) up to 250,000 Additional Commitment Fee Shares, and (iv)
55,000 SPA Commitment Fee Shares. The number of shares of our common stock ultimately offered for resale by the Selling Stockholders
under this prospectus is dependent upon the number of ELOC Shares issued. Depending on a variety of factors, including market liquidity
of our common stock, the issuance of shares to the Selling Stockholders may cause the trading price of our common stock to decline.
We
may not be able to comply with Nasdaq’s continued listing standards.
There
is no guarantee that we will be able to maintain our listing on Nasdaq for any period of time by perpetually satisfying Nasdaq’s
continued listing requirements. Our failure to continue to meet these requirements may result in our securities being delisted from Nasdaq.
At times, including during our 2023 fiscal year, we have received deficiency notices from Nasdaq regarding our inability to comply with
various of the continued listing rules (including stockholders’ equity requirements, publicly held share requirements, and timely
filing requirements). In the past we have taken steps to attempt to regain compliance with these listing rules, however, in the future
we may be unable to remain in compliance with Nasdaq’s continued listing requirements or remedy any deficiencies. If our common
stock were to be delisted from Nasdaq, it would likely reduce the liquidity of our common stock, and, among other things, may decrease
the attractiveness of our common stock to the investment community, and make it more difficult for us to issue equity securities for
capital raising purposes or for acquisitions.
Our
common stock has in the past been a “penny stock” under SEC rules, and may be subject to the “penny stock” rules
in the future. It may be more difficult to resell securities classified as “penny stock.”
In
the past (including immediately prior to our common stock being listed on Nasdaq in February 2020), our common stock was a “penny
stock” under applicable SEC rules (generally defined as non-exchange traded stock with a per-share price below $5.00). While our
common stock is not now considered a “penny stock” because it is listed on Nasdaq, if we are unable to maintain that listing,
unless we maintain a per-share price above $5.00, our common stock will become “penny stock.” These rules impose additional
sales practice requirements on broker-dealers that recommend the purchase or sale of penny stocks to persons other than those who qualify
as “established customers” or “accredited investors.” For example, broker-dealers must determine the appropriateness
for non-qualifying persons of investments in penny stocks. Broker-dealers must also provide, prior to a transaction in a penny stock
not otherwise exempt from the rules, a standardized risk disclosure document that provides information about penny stocks and the risks
in the penny stock market. The broker-dealer also must provide the customer with current bid and offer quotations for the penny stock,
disclose the compensation of the broker-dealer and its salesperson in the transaction, furnish monthly account statements showing the
market value of each penny stock held in the customer’s account, provide a special written determination that the penny stock is
a suitable investment for the purchaser, and receive the purchaser’s written agreement to the transaction.
Legal
remedies available to an investor in “penny stocks” may include the following:
| |
● |
If
a “penny stock” is sold to the investor in violation of the requirements listed above, or other federal or states securities
laws, the investor may be able to cancel the purchase and receive a refund of the investment. |
| |
|
|
| |
● |
If
a “penny stock” is sold to the investor in a fraudulent manner, the investor may be able to sue the persons and firms
that committed the fraud for damages. |
These
requirements may have the effect of reducing the level of trading activity, if any, in the secondary market for a security that becomes
subject to the penny stock rules. The additional burdens imposed upon broker-dealers by such requirements may discourage broker-dealers
from effecting transactions in our securities, which could severely limit the market price and liquidity of our securities. These requirements
may restrict the ability of broker-dealers to sell our common stock and may affect your ability to resell our common stock.
Many
brokerage firms will discourage or refrain from recommending investments in penny stocks. Most institutional investors will not invest
in penny stocks. In addition, many individual investors will not invest in penny stocks due, among other reasons, to the increased financial
risk generally associated with these investments.
For
these reasons, penny stocks may have a limited market and, consequently, limited liquidity. We can give no assurance at what time, if
ever, our common stock may be classified as a “penny stock” in the future.
The
exercise of outstanding warrants, options and other securities that are exercisable into shares of our common stock will be dilutive
to our existing stockholders.
As
of the date of this Registration Statement, we had outstanding various warrants, stock options and other securities that are exercisable
into shares of our common stock. For the life of the options and warrants, the holders have the opportunity to profit from a rise in
the market price of our common stock without assuming the risk of ownership. The issuance of shares upon the exercise of outstanding
securities will also dilute the ownership interests of our existing stockholders. The availability of these shares for public resale,
as well as any actual resales of these shares, could adversely affect the trading price of our common stock.
We
cannot predict the size of future issuances of our common stock pursuant to the exercise of outstanding options or warrants, or the effect,
if any, that future issuances and sales of shares of our common stock may have on the market price of our common stock. Sales or distributions
of substantial amounts of our common stock (including shares issued in connection with an acquisition), or the perception that such sales
could occur, may cause the market price of our common stock to decline.
We
have not historically paid or declared any dividends on our common stock and do not expect to pay or declare cash dividends in the future
on a regular basis, if at all.
Although
we declared special cash dividends in the first and third quarters of 2024, those dividends were declared as the result of a sale various
business assets and not paid from cash generated in our operations. The Company has not historically paid or declared any dividends on
our common stock or preferred stock. Any future dividends on common stock will be declared at the discretion of our board of directors
and will depend, among other things, on our earnings, our financial requirements for future operations and growth, and other facts as
we may then deem appropriate. As such, the return on your investment, if any, has historically been dependent solely on an increase,
if any, in the market value of our common stock.
Our
common stock price is likely to be highly volatile because of several factors, including a limited public float.
The
market price of our common stock has been volatile in the past and the market price of our common stock is likely to be highly volatile
in the future. You may not be able to resell shares of our common stock following periods of volatility because of the market’s
adverse reaction to volatility.
Other
factors that could cause such volatility may include, among other things:
| |
● |
actual
or anticipated fluctuations in our operating results; |
| |
|
|
| |
● |
the
absence of securities analysts covering us and distributing research and recommendations about us; |
| |
|
|
| |
● |
we
may have a low trading volume for a number of reasons, including that a large portion of our stock is closely held; |
| |
|
|
| |
● |
overall
stock market fluctuations; |
| |
|
|
| |
● |
announcements
concerning our business or those of our competitors; |
| |
|
|
| |
● |
actual
or perceived limitations on our ability to raise capital when we require it, and to raise such capital on favorable terms; |
| |
|
|
| |
● |
conditions
or trends in our industry; |
| |
|
|
| |
● |
litigation; |
| |
● |
changes
in market valuations of other similar companies; |
| |
|
|
| |
● |
future
sales of common stock; |
| |
|
|
| |
● |
departure
of key personnel or failure to hire key personnel; and |
| |
|
|
| |
● |
general
market conditions. |
Any
of these factors could have a significant and adverse impact on the market price of our common stock. In addition, the stock market in
general has at times experienced extreme volatility and rapid decline that has often been unrelated or disproportionate to the operating
performance of particular companies. These broad market fluctuations may adversely affect the trading price of our common stock, regardless
of our actual operating performance.
There
may not be sufficient liquidity in the market for our securities in order for investors to sell their shares. The market price of our
common stock may continue to be volatile.
The
market price of our common stock will likely continue to be highly volatile. Some of the factors that may materially affect the market
price of our common stock are beyond our control, such as conditions or trends in the industry in which we operate or sales of our common
stock. This situation is attributable to a number of factors, including the fact that we are a small company which is relatively unknown
to stock analysts, stock brokers, institutional investors and others in the investment community that generate or influence sales volume,
and that even if we came to the attention of such persons, they tend to be risk-averse and would be reluctant to follow an unproven company
such as ours or purchase or recommend the purchase of our shares until such time as we became more seasoned and viable.
As
a consequence, there may be periods of several days or more when trading activity in our shares is minimal or non-existent, as compared
to a mature issuer which has a large and steady volume of trading activity that will generally support continuous sales without an adverse
effect on share price. It is possible that a broader or more active public trading market for our common stock will not develop or be
sustained, or that trading levels will not continue. These factors may materially adversely affect the market price of our common stock,
regardless of our performance. In addition, the public stock markets have experienced extreme price and trading volume volatility. This
volatility has significantly affected the market prices of securities of many companies for reasons frequently unrelated to the operating
performance of the specific companies. These broad market fluctuations may adversely affect the market price of our common stock.
Stockholders
may be diluted significantly through our efforts to obtain financing and satisfy obligations through the issuance of additional shares
of our common stock.
Wherever
possible, our board of directors will attempt to use non-cash consideration to satisfy obligations. In many instances, we believe that
the non-cash consideration will consist of restricted shares of our common stock or where shares are to be issued to our officers, directors
and applicable consultants. Our board of directors has authority, without action or vote of the stockholders, but subject to Nasdaq rules
and regulations (which generally require shareholder approval for any transactions which would result in the issuance of more than 20%
of our then outstanding shares of common stock or voting rights representing over 20% of our then outstanding shares of stock), to issue
all or part of the authorized but unissued shares of common stock. In addition, we may attempt to raise capital by selling shares of
our common stock, possibly at a discount to market. These actions will result in dilution of the ownership interests of existing stockholders,
which may further dilute common stock book value, and that dilution may be material. Such issuances may also serve to enhance existing
management’s ability to maintain control of the Company because the shares may be issued to parties or entities committed to supporting
existing management.
USE
OF PROCEEDS
The
Selling Stockholders will receive all of the proceeds of the sale of shares of common stock offered from time to time pursuant
to this prospectus. Accordingly, we will not receive any proceeds from the sale of shares of common stock that may be sold from time
to time pursuant to this prospectus.
We
may receive up to $50,000,000 in aggregate gross proceeds under the ELOC Purchase Agreement in connection with sales of our shares of
common stock to Arena Global that we may, in our discretion, elect to make, from time to time pursuant to the ELOC Purchase Agreement
after the date of this prospectus. We intend to use the proceeds from the sale of our shares of common stock to Arena Global for
general corporate and working capital purposes. Our management will have broad discretion over the use of proceeds from the sale of our
shares of common stock under the ELOC Purchase Agreement.
More
specifically, we intend to use the net proceeds from the sale of our shares of common stock under the ELOC Purchase Agreement to fund
(i) continued research and development, clinical development, and regulatory approvals of our products; (ii) costs associated with seeking
and maintaining intellectual property protections of our products; (iii) our commercial operations, including building out a sales and
marketing infrastructure through contract partners and service providers; (iv) the expansion of our product portfolio through strategic
third party in-licensing and synergistic acquisitions of product assets that can leverage our commercial infrastructure; (v) the strategic
expansion of our leadership team through recruitment of experienced team members; and (vi) to pay down certain outstanding debt obligations
and settle payments coming due. These intended uses are summarized in the following table:
| Function | |
Product | |
2025 | | |
2026 | | |
2027 | | |
Total | |
| G&A | |
| |
$ | 1,908,400 | | |
$ | 2,236,000 | | |
$ | 2,478,400 | | |
$ | 6,622,800 | |
| R&D/Clinical/ Regulatory | |
| |
$ | 5,690,000 | | |
$ | 6,178,000 | | |
$ | 11,593,000 | | |
$ | 23,461,000 | |
| | |
SCN-102(1) | |
$ | 405,000 | | |
$ | 838,000 | | |
$ | 838,000 | | |
$ | 2,081,000 | |
| | |
SCN-104(2) | |
$ | 3,895,000 | | |
$ | 2,250,000 | | |
$ | 405,000 | | |
$ | 6,550,000 | |
| | |
SCN-106(3) | |
$ | 890,000 | | |
$ | 2,090,000 | | |
$ | 9,350,000 | | |
$ | 12,330,000 | |
| | |
SCN-107(4) | |
$ | 500,000 | | |
$ | 1,000,000 | | |
$ | 1,000,000 | | |
$ | 2,500,000 | |
| Commercial(5) | |
| |
$ | 3,318,295 | | |
$ | 5,018,295 | | |
$ | 6,218,295 | | |
$ | 14,554,885 | |
| Intellectual Property(6) | |
| |
$ | 80,000 | | |
$ | 100,000 | | |
$ | 150,000 | | |
$ | 330,000 | |
| Business Development(7) | |
| |
$ | 600,000 | | |
$ | 1,000,000 | | |
$ | 1,000,000 | | |
$ | 2,600,000 | |
| One Time Convertible Debt Payment | |
| |
$ | 2,865,000 | | |
$ | — | | |
$ | — | | |
$ | 2,865,000 | |
| One Time Settlement | |
| |
$ | 985,000 | | |
$ | 300,000 | | |
$ | — | | |
$ | 1,285,000 | |
| | |
Total | |
$ | 15,446,695 | | |
$ | 14,832,295 | | |
$ | 21,439,695 | | |
$ | 51,718,685 | |
(1)
The Company anticipates using a portion of the net proceeds to fund NDA approval and the launch of SCN-102.
(2)
The Company anticipates using a portion of the net proceeds to fund NDA filing costs associated with SCN-104.
(3)
The Company anticipates using a portion of the net proceeds to fund process development and optimization of SCN-106.
(4)
The Company anticipates using a portion of the net proceeds to fund the scale-up of, and preclinical activities associated with, SCN-107.
(5)
The Company anticipates using a portion of the net proceeds to improve and implement its commercial infrastructure.
(6)
The Company anticipates using a portion of the net proceeds to pay for certain expenses associated with obtaining and maintaining patent
protection for its product portfolio.
(7)
The Company anticipates using a portion of the net proceeds to fund the in-licensing and launch of its product portfolio.
Our
expected use of net proceeds from the sale of our shares of common stock under the ELOC Purchase Agreement represents our current intentions
based upon our present plans and business condition. We reserve the right to change the foregoing use of proceeds, should our management
believe it to be in the best interest of our company.
As
of the date of this prospectus, we cannot currently allocate specific percentages of the net proceeds that we may use for the purposes
specified above, and we cannot predict with certainty all of the particular uses for the net proceeds to be received pursuant to the
ELOC Purchase Agreement, or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual
expenditures will depend upon numerous factors, including our sales and marketing efforts, demand for our products, our operating costs
and the other factors described under and incorporated by reference in “Risk Factors” in this prospectus. Accordingly,
our management will have flexibility in applying the net proceeds from the sale of our shares of common stock under the ELOC Purchase
Agreement. An investor will not have the opportunity to evaluate the economic, financial or other information on which we base our decisions
on how to use the proceeds. In the event we do not obtain the entire amount pursuant to the ELOC Purchase Agreement, we may attempt to
obtain additional funds through other offerings of our securities or by borrowing funds.
SELLING
STOCKHOLDERS
The
Selling Stockholders listed in the table below may from time to time offer and sell any or all of the shares set forth below pursuant
to this prospectus. When we refer to the Selling Stockholders in this prospectus, we refer to each entity listed in the
table below, and the pledgees, donees, transferees, assignees, successors and other permitted transferees that hold any of the Selling
Stockholders’ interest in the shares after the date of this prospectus.
The
following table sets forth certain information provided by or on behalf of the Selling Stockholders concerning the shares that
may be offered from time to time by the Selling Stockholders pursuant to this prospectus. The Selling Stockholders identified
below may have sold, transferred or otherwise disposed of all or a portion of their shares or other Company securities after the date
on which they provided us with information regarding such securities. Moreover, the shares identified below include only the shares being
registered for resale and may not incorporate all shares of common stock or other securities of the Company deemed to be beneficially
held by the Selling Stockholders. Any changed or new information given to us by the Selling Stockholders, including regarding
the identity of, and the securities held by, the Selling Stockholders, will be set forth in a prospectus supplement or amendments
to the registration statement of which this prospectus is a part, if and when necessary. The Selling Stockholders may sell all,
some or none of the shares in this offering. See “Plan of Distribution.”
Other
than as described below or elsewhere in this prospectus, the Selling Stockholders do not have any material relationship with
us or any of our predecessors or affiliates.
The
number of shares of common stock beneficially owned by each Selling Stockholder is determined under rules promulgated by the SEC.
Name and address of Selling Stockholder | |
Number
of Shares Owned Prior to
the Offering | | |
Maximum Number
of
Shares to be
Sold Pursuant to this Prospectus | | |
Number of Shares
Owned After Offering
(1) | | |
Percent of Shares
Owned After
Offering (1) | |
| | |
| | | |
| | | |
| | | |
| | |
| Arena Finance Markets, LP (2) | |
| 44,703 | | |
| 44,703 | | |
| — | | |
| — | |
Arena Special Opportunities Partners III, LP (3) | |
| 10,297 | | |
| 10,297 | | |
| — | | |
| — | |
| Arena Business Solutions Global SPC II, Ltd (4) | |
| 891,193 | (5) | |
| 4,245,000 | | |
| — | | |
| — | |
| (1) |
Assumes
that the Selling Stockholders will sell all of the shares offered by them under this prospectus. |
| |
|
| (2) |
The
principal business address for Arena Finance is 405 Lexington Ave, 59th Floor, New York, NY 10174. Dan Zwirn has voting and dispositive power over the shares owned by Arena
Finance. |
| |
|
| (3) |
The
principal business address for ASOP is 405 Lexington Ave, 59th Floor, New York, NY 10174. Dan Zwirn has voting and dispositive power over the shares owned by ASOP. |
| |
|
| (4) |
The
principal business address for Arena Global is 405 Lexington Ave, 59th Floor, New York, NY 10174. Dan Zwirn has voting and dispositive
power over the shares owned by Arena Global. |
| |
|
| (5) |
Total
shares owned (i) assumes the issuance of all shares of common stock under the ELOC Purchase
Agreement and (ii) gives effect to the 9.99% Beneficial Ownership Limitation. |
DESCRIPTION
OF eloc PURCHASE AGREEMENT
On
November 25, 2024, we entered into the ELOC Purchase Agreement with Arena Global, pursuant to which we have the right, but not
the obligation, to direct Arena Global to purchase up to $50,000,000 in ELOC Shares upon satisfaction of certain terms and conditions
contained in the ELOC Purchase Agreement. Such sales of our common stock, if any, will be subject to certain limitations, and may occur
from time to time at our sole discretion over the approximately 36-month period commencing on the date of execution of the ELOC Purchase
Agreement, provided that the registration statement of which this prospectus forms a part, and any other registration statement the Company
may file from time to time covering the resale by Arena Global of ELOC Shares or Commitment Fee Shares, is declared effective by the
SEC and remains effective, and the other conditions set forth in the ELOC Purchase Agreement are satisfied.
Arena
Global has no right to require any sales by us, but Arena Global is obligated to make purchases at our direction subject to certain conditions.
There is no upper limit on the price per share that Arena Global could be obligated to pay for ELOC Shares under the ELOC Purchase Agreement.
Actual sales of ELOC Shares to Arena Global from time to time will depend on a variety of factors, including, among others, market conditions,
the trading price of our common stock and determinations by us as to the appropriate sources of funding for us and our operations. The
net proceeds that we may receive under the ELOC Purchase Agreement, if any, cannot be determined at this time, since it will depend on
the frequency and prices at which we sell ELOC Shares to Arena Global, our ability to meet the conditions of the ELOC Purchase Agreement,
and the other limitations, terms and conditions of the ELOC Purchase Agreement and any impacts of the Beneficial Ownership Limitation.
The
ELOC Purchase Agreement contains customary representations, warranties, conditions and indemnification obligations of the parties.
Purchase
of ELOC Shares
Under
the ELOC Purchase Agreement, after the satisfaction of certain conditions, we have the right to present Arena Global with an Advance
Notice directing it to purchase any amount of ELOC Shares, up to the Maximum Advance Amount, which is calculated as follows:
| (a) | if
the Advance Notice is received by 8:30 a.m. Eastern Time, the lower of: (i) an amount equal
to eighty percent (80%) of the average of the Daily Value Traded (as defined in the ELOC
Purchase Agreement) of our common stock on the ten (10) trading days immediately preceding
an Advance Notice, or (ii) $20,000,000; |
| | | |
| (b) | if
the Advance Notice is received after 8:30 a.m. Eastern Time but prior to 10:30 a.m. Eastern
Time, the lower of: (i) an amount equal to forty percent (40%) of the average of the Daily
Value Traded of our common stock on the ten (10) trading days immediately preceding an Advance
Notice, or (ii) $10,000,000; |
| | | |
| (c) | if
the Advance Notice is received after 10:30 a.m. Eastern Time, but prior to 12:30 p.m. Eastern
Time, the lower of: (i) an amount equal to twenty percent (20%) of the average of the Daily
Value Traded of our common stock on the ten (10) trading days immediately preceding an Advance
Notice, or (ii) $5,000,000; and |
| | | |
| (d) | if
the Advance Notice is received after 12:30 p.m. Eastern Time but prior to 2:30 p.m. Eastern
Time, the lower of: (i) an amount equal to ten percent (10%) of the average of the Daily
Value Traded of our common stock on the ten (10) trading days immediately preceding an Advance
Notice, or (ii) $2,500,000. |
The
purchase price to be paid by Arena Global for the ELOC Shares will be ninety-six percent (96%) of the VWAP (as defined in the ELOC Purchase
Agreement) of the Company’s common stock during the trading day commencing on the date of the Advance Notice, subject to adjustment
pursuant to the terms of the ELOC Purchase Agreement.
Consideration
In
consideration for Arena Global’s execution and delivery of the ELOC Purchase Agreement, we agreed to issue to Arena Global, as
a commitment fee: (i) 70,000 Initial Commitment Fee Shares and (ii) in two separate tranches, a number of Additional Commitment Fee Shares
equal to (a) the First Trance Price and (b) the Second Trance Price. The Additional Commitment Fee Shares shall be subject to a true-up
after each issuance pursuant to the terms of the ELOC Purchase Agreement. In connection with the true-up, we will issue to Arena Global
a number of additional shares of our common stock, if any, having an aggregate dollar value equal to:
| (i) | with
respect to the first tranche, 500,000 based on the lower of (A) the First Tranche Price and
(B) the lower of (x) the simple average of the three (3) lowest daily intraday trade prices
over the twenty (20) trading days after (and not including) the Effectiveness Date and (y)
the closing price on the twentieth (20th) trading day after the Effectiveness Date; and |
| | | |
| (ii) | with
respect to the second tranche, 500,000 based on the lower of (A) the Second Tranche Price
and (B) the lower of (x) the simple average of the three (3) lowest daily intraday trade
prices over the twenty (20) trading days after (and not including) the Anniversary and (y)
the closing price on the twentieth (20th) trading day after the Anniversary. |
Conditions
to Delivery of Advance Notices
Our
ability to deliver Advance Notices under the EOLC Purchase Agreement is subject to the satisfaction of certain conditions, including,
among other things, the following:
| ● | the
representations and warranties of the Company under the ELOC Purchase Agreement being true
and correct in all material respects; |
| | | |
| ● | the
effectiveness of that the registration statement of which this prospectus forms a part, and
any other registration statement the Company may file from time to time covering the resale
by Arena Global of ELOC Shares or ELOC Commitment Fee Shares; |
| | | |
| ● | the
Company having filed with the SEC all reports, notices and other documents required under
the Exchange Act and applicable SEC regulations during the six-month period immediately preceding
the delivery of the Advance Notice; |
| | | |
| ● | the
Company having obtained all permits and qualifications required by any applicable state for
the offer and sale of all the ELOC Shares issuable pursuant to the Advance Notice, or having
the availability of exemptions therefrom; |
| | | |
| ● | no
continuing Material Outside Event or Material Adverse Effect (each as defined in the ELOC
Purchase Agreement) having occurred; |
| | | |
| ● | the
Company having performed, satisfied and complied in all material respects with all covenants,
agreements and conditions required by the ELOC Purchase Agreement to be performed, satisfied
or complied with by the Company at or prior the delivery of the Advance Notice, including,
without limitation, the delivery of all ELOC Shares issuable pursuant to all previously delivered
Advance Notices and the issuance of all ELOC Commitment Fee Shares previously required
to be issued; |
| | | |
| ● | no
statute, rule, regulation, executive order, decree, ruling or injunction having been enacted,
entered, promulgated or endorsed by any court or governmental authority of competent jurisdiction
that prohibits or directly, materially and adversely affects any of the transactions contemplated
by the ELOC Purchase Agreement; |
| | | |
| ● | the
Company’s shares of common stock, including all of the ELOC Shares issuable pursuant
to the Advance Notice, being quoted for trading on Nasdaq; |
| | | |
| ● | the
Company shall not have received any written notice that is then still pending threatening
the continued quotation of the common stock on Nasdaq; |
| ● | the
Company having a sufficient number of authorized but unissued, and otherwise unreserved,
shares of common shares available for the issuance of all of the ELOC Shares issuable pursuant
to the Advance Notice; |
| | | |
| ● | the
representations contained in the Advance Notice being true and correct in all material respects
as of the date of delivery of the Advance Notice; |
| | | |
| ● | except
with respect to the first Advance Notice, the Pricing Period (as defined in the ELOC Purchase
Agreement) for all prior Advance Notices having been completed; and |
| | | |
| ● | the
Company having obtained shareholder approval to issue an aggregate number of shares of common
stock to Arena Global, under the ELOC Purchase Agreement, in excess of 19.99% of the number
of shares of common stock outstanding immediately prior to the execution of the ELOC Purchase
Agreement. |
Limitation
on Sales
At
any given time of any sale by us to Arena Global, we may not sell, and Arena Global may not purchase, ELOC Shares that would result in
Arena Global owning more than the 9.99% Beneficial Ownership Limitation.
No
Short Selling or Hedging by Arena Global
Arena
Global has agreed that, during the term of the ELOC Purchase Agreement, neither Arena Global nor any of its affiliates will engage in
any short sales or hedging transactions with respect to our common stock.
Termination
of the ELOC Purchase Agreement
Unless
earlier terminated as provided in the ELOC Purchase Agreement, the ELOC Purchase Agreement will terminate automatically on the earliest
to occur of: (i) December 1, 2027, (ii) the date on which Arena Global shall have purchased the maximum amount of ELOC Shares, or (iii)
the effective date of any written notice of termination delivered pursuant to the terms of the ELOC Purchase Agreement.
Limitation
on Variable Rate Transactions
Pursuant
to the ELOC Purchase Agreement, from the date of the ELOC Purchase Agreement until the earlier of (i) the date that Arena Global has
purchased $20,000,000 in ELOC Shares, (ii) twelve (12) months after the Effectiveness Date, or (iii) three (3) months after the termination
of the ELOC Purchase Agreement, pursuant to its terms, the Company is prohibited from effecting or entering into an agreement to effect
any issuance of our common stock or common stock equivalents involving a Variable Rate Transaction (as defined in the ELOC Purchase Agreement),
other than in connection with an Exempt Issuance (as defined in the ELOC Purchase Agreement) or with the prior written consent of Arena
Global.
Dilutive Effect
All
shares of common stock registered in this offering which have been or may be issued or sold by us to Arena Global under the ELOC Purchase
Agreement are expected to be freely tradable. It is anticipated that the shares of common stock registered in this offering will be sold
over a period starting on the date that the registration statement of which this prospectus is a part is declared effective and ending
on December 1, 2027. The sale by Arena Global of a significant amount of common stock registered in this offering could cause the market
price of our common stock to decline and to be highly volatile. Sales of ELOC Shares to Arena Global, if any, will depend upon market
conditions and other factors to be determined by us. We may ultimately decide to sell to Arena Global all, some or none of the ELOC Shares.
Global
may resell all, some or none of the shares of common stock held by it at any time, or from time to time, in its discretion. Therefore,
sales to Arena Global by us under the ELOC Purchase Agreement may result in substantial dilution to the interests of other holders of
common stock. In addition, if we sell a substantial number of ELOC Shares to Arena Global, or if investors expect that we will do so,
the actual sales of ELOC Shares or the mere existence of our arrangement with Arena Global may make it more difficult for us to sell
securities in the future at a time and at a price that we might otherwise wish to effect such sales. However, we have the right to control
the timing and amount of any sales to Arena Global and we are not obligated to submit any Advance Notices under the ELOC Purchase Agreement.
The following
table sets forth the amount of gross proceeds we would receive from Arena Global from the sale of ELOC Shares to Arena Global under the
ELOC Purchase Agreement at varying purchase prices, without giving effect to the Beneficial Ownership Limitation, for illustrative purposes
only. The Beneficial Ownership Limitation may not be increased above 9.99% of our then outstanding common stock. Furthermore,
as noted above, we are not obligated to submit any Advance Notices under the ELOC Purchase Agreement.
Assumed
Average
Purchase
Price Per Share (1) |
|
|
Number
of Registered
Common Shares to be
Issued if Full Purchase,
Without Giving Effect to the
Beneficial Ownership Limitation (2) |
|
|
Percentage
of Outstanding
Common Stock After Giving
Effect to the Issuance to
Arena Global, Without
Giving Effect to the Beneficial
Ownership
Limitation (3) |
|
|
Proceeds
from the Sale of
Common Stock to Arena
Global Under the ELOC
Purchase Agreement(4) |
|
| $ |
6.00 |
|
|
|
8,333,333 |
|
|
|
48.8 |
% |
|
$ |
50,000,000 |
|
| $ |
7.00 |
|
|
|
7,142,857 |
|
|
|
45.0 |
% |
|
$ |
50,000,000 |
|
| $ |
7.4112 |
(5) |
|
|
6,746,545 |
|
|
|
43.6 |
% |
|
$ |
50,000,000 |
|
| $ |
8.00 |
|
|
|
6,250,000 |
|
|
|
41.7 |
% |
|
$ |
50,000,000 |
|
| $ |
8.50 |
|
|
|
5,882,353 |
|
|
|
40.3 |
% |
|
$ |
50,000,000 |
|
| (1) |
For the avoidance of any doubt, this price reflects the purchase
price after calculation (i.e. after discounts to the market price of our shares) in accordance with the terms of the ELOC Purchase
Agreement. |
| |
|
| (2) |
Represents the number of ELOC Shares that could potentially be issued
to Arena Global during the Commitment Period, in the aggregate, based on the applicable assumed purchase price per share, without
giving effect to the Beneficial Ownership Limitation. |
| |
|
| (3) |
The denominator is based on 8,730,366 shares of our common stock
outstanding as of December 3, 2024, adjusted to include the issuance of the number of shares of common stock set forth in
the adjacent column which we would have issued to Arena Global based on the applicable assumed purchase price per share. |
| |
|
| (4) |
The Company did not receive any proceeds from the issuance of the
ELOC Commitment Fee Shares to Arena Global. |
| |
|
| (5) |
Represents the last reported sales price of our common stock on
December 2, 2024, as reported by Nasdaq, less a four percent (4%) discount. |
The
ELOC Shares and ELOC Commitment Fee Shares were, and will be, offered and sold to Arena Global in reliance upon the exemption
from registration provided by Section 4(a)(2) of the Securities Act.
The
foregoing summary of the ELOC Purchase Agreement is qualified in its entirety by reference to the full text of the ELOC Purchase Agreement,
which is incorporated by reference as an exhibit to the registration statement of which this prospectus forms a part.
DESCRIPTION
OF SECURITIES PURCHASE AGREEMENT
On
November 22, 2024, the Company entered into the Securities Purchase Agreement with the Arena Investors. Under the Securities Purchase
Agreement, the Company will issue Debentures in a principal amount of up to $12,222,222 million, divided into up to three separate tranches
that are each subject to certain closing conditions. The conversion price per share of each Debenture is equal to 92.5% of the lowest
daily VWAP (as defined in the Debentures) of the Company’s shares of common stock during the five trading day period ending on
the trading day immediately prior to delivery or deemed delivery of the applicable Conversion Notice (as defined in the Debentures),
subject to adjustments related to the trading price of the Company’s common stock.
The
First Closing was consummated on November 25, 2024 and the Company issued to the Arena Investors the First Closing Debentures in an aggregate
principal amount of $3,333,333. The First Closing Debentures were sold to the Arena Investors for a purchase price of $3,000,000, representing
an original issue discount of ten percent (10%).
The
First Closing Debentures contain customary events of default. If an event of default occurs, until it is cured, the holder may increase
the interest rate applicable to the First Closing Debentures to two percent (2%) per annum and accelerate the full indebtedness under
the First Closing Debentures, in an amount equal to 125% of the outstanding principal amount and accrued and unpaid interest. Subject
to limited exceptions set forth in the First Closing Debentures, the First Closing Debentures prohibit the Company and, as applicable,
its subsidiaries from incurring any new indebtedness that is not subordinated to the Arena Investors and, as applicable, any subsidiary’s
obligations in respect of the First Closing Debentures until the First Closing Debentures are paid in full.
As
consideration for the Arena Investors’ consummation of the First Closing, concurrently with the First Closing, the Company issued
to each Arena Investor participating in the First Closing its pro rata portion of the 55,000 SPA Commitment Fee Shares. Furthermore,
as consideration for the Arena Investors’ consummation of subsequent closings, the Company shall issue to the Arena Investors participating
in such closing a certain number of Company common stock as agreed upon among the Company and the Arena Investors participating.
The
Company agreed, pursuant to the Security Agreement, to grant the Arena Investors a security interest in all of its assets to secure the
prompt payment, performance, and discharge in full of all of the Company’s obligations under the Debentures. In addition, the Company’s
wholly-owned subsidiary, Scienture LLC, entered into the Guarantee Agreement, pursuant to which it agreed to guarantee the prompt payment,
performance, and discharge in full of all of the Company’s obligations under the Debentures.
The
Company also agreed, pursuant to the Registration Rights Agreement, to file with the SEC an initial registration statement within 45
days to register the maximum number of Registrable Securities (as defined in the Registration Rights Agreement) in accordance with applicable
SEC rules.
The
Securities Purchase Agreement, Debentures, Security Agreement, Guaranty Agreement, and Registration Rights Agreement contain customary
representations, warranties, agreements and conditions to completing future sale transactions, indemnification rights and obligations
of the parties. Among other things, the Arena Investors represented to the Company, that they are each an “accredited investor”
(as such term is defined in Rule 501(a) of Regulation D under the Securities Act). The Company issued, and will issue, the securities
in reliance upon an exemption from registration contained in Section 4(a)(2) of the Securities Act and Regulation D promulgated thereunder.
DESCRIPTION
OF BUSINESS
Corporate
and Organizational History
Background
of XCEL
We
were incorporated in Delaware on July 15, 2005, as “Bluebird Exploration Company” (“Bluebird”). Bluebird was
originally formed to engage in the exploitation of mineral properties. In December 2008, Bluebird changed its name to “Xcellink
International, Inc.” (“XCEL”), and subsequently announced that its business plan was being expanded to include the
development and marketing of platform-independent customer-centric payment systems and methodologies. XCEL was unable to raise the funds
necessary to implement its business strategy, and never generated any revenue. On January 9, 2014, Trxade Group, Inc., a then privately
held Nevada corporation, merged with and into XCEL, and XCEL changed its name to “Trxade Group, Inc.” On June 1, 2021, the
Company changed its name from “Trxade Group, Inc” to “TRxADE HEALTH, Inc.” On September 20, 2024, the Company
changed its name from “TRxADE HEALTH, Inc.” to “Scienture Holdings, Inc.”
Background
of Trxade
PharmaCycle
LLC, a Nevada limited liability company (“PharmaCycle”), was formed in August 2010 by Prashant Patel, our President, Chief
Operating Officer, and Interim, to serve as a web-based market platform designed to enable trading among healthcare buyers and sellers
of pharmaceuticals, accessories and services. In January 2013, PharmaCycle converted into a Florida corporation and changed its name
to Trxade, Inc. (“Trxade Florida”). In May 2013, Trxade Florida created a new wholly-owned subsidiary, Trxade Group, Inc.,
a Nevada corporation (“Trxade Nevada”). Trxade Nevada acquired Trxade Florida pursuant to a reverse triangular merger, resulting
in Trxade Florida becoming a wholly-owned subsidiary of Trxade Nevada (the “Nevada-Florida Merger”). The sole purpose of
the Nevada-Florida Merger was to provide for a holding company to own Trxade Florida, the operating company. Immediately following the
Nevada-Florida Merger, Messrs. Ajjarapu and Patel collectively owned 99% of Trxade Nevada.
Reverse
Merger with Trxade
On
September 26, 2008, Mark Fingarson, the former President, sole Director and controlling shareholder of XCEL, sold 80,000,000 shares of
XCEL (prior to the Merger Reverse Split and Reverse Stock Splits (each discussed and defined below)). On November 22, 2013, Trxade Nevada
acquired Mr. McIntyre’s controlling interest of 80,000,000 shares in XCEL pursuant to a Purchase and Sale Agreement dated November
7, 2013. At the time of the sale, XCEL had 104,160,000 shares of common stock issued and outstanding, including the 80,000,000 shares
of stock acquired by Trxade Nevada (prior to the Merger Reverse Split and Reverse Stock Split(s) (each discussed and defined below)).
On
December 16, 2013, Trxade Nevada and XCEL entered into a definitive merger agreement (the “Merger Agreement”) providing for
the merger (the “Merger”) of Trxade Nevada with and into XCEL, with XCEL continuing as the surviving corporation. The Merger
closed on January 8, 2014. Under the terms of the Merger Agreement, we amended our certificate of incorporation and changed our name
to “Trxade Group, Inc.,” and changed our trading symbol to “TRXD”.
Recapitalization
of Common Stock by a Reverse Split and Increase of Authorized Shares of Stock
We
also reversed our issued and outstanding stock at the ratio of one for one thousand (1:1,000) shares effective upon the closing of the
Merger (the “Merger Reverse Split”). In connection with the Merger Reverse Split, 104,160,000 outstanding shares of our common
stock, including the 80,000,000 shares held by Trxade Nevada, were exchanged for 104,160 post-Merger Reverse Split shares of common stock.
As a result of the Merger, Trxade Nevada stockholders holding 28,800,000 shares of common stock and 670,000 shares of Series A Preferred
Stock converted their shares on a one-to-one basis into 28,800,000 shares of our common stock and 670,000 shares of our Series A Preferred
Stock, for an aggregate total of 29,470,000 shares. Further, 100,000 shares of our common stock (on a post-Reverse Split basis and considering
the Reverse Stock Split(s) (discussed below)) were issued following the Merger in connection with the conversion of our promissory notes.
The 80,000,000 pre-Merger shares held by Trxade Nevada, which amounted to 13,334 shares (on a post-Reverse Split basis and taking into
account the Reverse Stock Split(s)), reverted to treasury stock of the Company. Except as otherwise disclosed, the share amounts in the
paragraph above have not been adjusted for the Merger Reverse Split or the Reverse Stock Split.
February
2020 Reverse Stock Split and NASDAQ Capital Market Listing
In
February 2020, the Company effected a 1-for-6 reverse stock split of the then outstanding common stock in order to allow us to meet the
initial listing criteria of Nasdaq.
Our
common stock was approved for listing on Nasdaq under the symbol “MEDS”, on February 13, 2020.
June
2023 Reverse Stock Split.
In
June 2023 the Company effected a 1-for-15 reverse stock split of its issued and outstanding common stock.
Subsidiaries
We
currently own 100% of Scienture LLC, Softell Inc. (f/k/a Trxade, Inc.), IPS, Bonum Health, Inc., and Bonum Health. During the year ended
December 31, 2023, and a portion of the quarter ended March 31, 2024, Softell, operated a web-based market platform that enables commerce
among healthcare buyers and sellers of pharmaceuticals, accessories and services.
Scienture
LLC is a specialty pharmaceutical company focused on developing and commercializing products for the treatment of central nervous system
(“CNS”) and cardiovascular (“CVS”) diseases.
IPS
is a licensed pharmaceutical wholesaler and sells brand, generic and non-drug products to customers. IPS customers include all healthcare
markets including government organizations, hospitals, clinics and independent pharmacies nationwide.
Bonum
Health was formed to hold certain telehealth assets acquired in October 2019. The “Bonum Health Hub” was launched in February
2020; however, the Company does not anticipate installations moving forward. We currently anticipate dissolving Bonum Health, Inc. and
Bonum Health.
On
October 4, 2024, the Company and Softell Inc. (f/k/a Trxade, Inc.) (“Softell”) entered into an Assignment and Assumption
of Membership Interests (the “IPS Assignment Agreement”), pursuant to which the Company transferred, and Softell accepted,
100% of the membership interests of IPS. As a result, IPS is now a wholly-owned subsidiary of Softell. During the year ended December
31, 2023 and a portion of the quarter ended March 31, 2024, Softell, operated a web-based market platform that enabled commerce among
healthcare buyers and sellers of pharmaceuticals, accessories and services. Softell’s current primary operations are conducted
through IPS.
Superlatus
Merger
On
July 14, 2023, the Company entered into an Amended and Restated Agreement and Plan of Merger (the “Superlatus Merger Agreement”)
with Superlatus and Foods Merger Sub, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub”).
Superlatus
is a diversified food technology company with distribution capabilities and systems to optimize food security and population health via
innovative Consumer Packaged Goods (“CPG”) products, agritech, foodtech, plant-based proteins and alt-protein and includes
wholly-owned subsidiary, Sapientia, Inc. (“Sapientia”), a food tech business.
On
July 31, 2023, the Company completed its acquisition of Superlatus in accordance with the terms and conditions of the Superlatus Merger
Agreement (the “Superlatus Merger”), pursuant to which the Company acquired Superlatus by way of a merger of the Merger Sub
with and into Superlatus, with Superlatus being a wholly owned subsidiary of the Company and the surviving entity in the Superlatus Merger.
Under
the terms of the Superlatus Merger Agreement, at the closing of the Superlatus Merger (the “Closing”), shareholders of Superlatus
received an aggregate of 136,441 shares of the Company’s common stock and 306,855 shares of the Company’s Series B Preferred
Stock, par value $0.00001 per share (the “Series B Preferred Stock”), convertible into 100 shares of the Company’s
common stock. At Closing, the value of the Company’s common stock was $7.30 per share, resulting in a total value of $225,000,169.
Upon consummation of the Superlatus Merger, the Company continued to trade under the former ticker symbol “MEDS.”
Not
all of the closing conditions of the Superlatus Merger Agreement were met. As a result, the Company entered into Amendment No. 1 to the
Amended and Restated Agreement and Plan of Merger (the “Superlatus Amendment”) on January 8, 2024. Under the terms of the
Superlatus Amendment, the merger consideration to the shareholders of Superlatus was adjusted to the aggregate of 136,441 shares of the
Company’s common stock and 15,759 shares of the Company’s Series B Preferred Stock, resulting in a total value of $12,500,089.
Additionally, the shareholders of Superlatus agreed to surrender back to the Company 291,096 shares of the Company’s Series B Preferred
Stock. As described below, the Company divested of its interest in Superlatus in March 2024.
Scienture
Merger
On
July 25, 2024, the Company entered into and closed an Agreement and Plan of Merger (the “Scienture Merger Agreement”) with
MEDS Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub I”), MEDS Merger
Sub II, LLC, a Delaware limited liability company and wholly owned subsidiary of the Company (“Merger Sub II” and, together
with Merger Sub I, the “Merger Subs”), and Scienture LLC. Pursuant to the Scienture Merger Agreement, (i) Merger Sub I merged
with and into Scienture LLC (the “First Merger”), with Scienture LLC continuing as the surviving entity and a wholly owned
subsidiary of the Company, and (ii) Scienture LLC merged with and into Merger Sub II (the “Second Merger” and, together with
the First Merger and all other related transactions, the “Scienture Merger”), with Merger Sub II continuing as the surviving
entity. In connection with the transactions, the Company changed its name to “Scienture Holdings, Inc.” and Merger Sub II,
as the surviving entity of the Second Merger, changed its name to “Scienture, LLC”.
As
consideration for the Scienture Merger, at the effective time of the First Merger (the “Effective Time”), the shares of Scienture
LLC common stock issued and outstanding immediately prior to the Effective Time were converted into the right to receive, in the aggregate,
(i) 291,536 shares of the Company’s common stock and (ii) 6,826,753 shares of the Company’s Series X Non-Voting Convertible
Preferred Stock (the “Series X Preferred Stock”), each share of which is convertible into one share of common stock.
Dispositions
SOSRx,
LLC
SOSRx,
LLC (“SOSRx”) was formed on February 15, 2022. The Company entered into a relationship with Exchange Health, LLC (“Exchange
Health”), a technology company providing an online platform for manufacturers and suppliers to sell and purchase pharmaceuticals.
SOSRx, a Delaware limited liability company, was formed, which was owned 51% by the Company and 49% by Exchange Health. SOSRx did not
generate material revenue and in February of 2023, the Company voluntarily withdrew from the joint venture agreement.
Community
Specialty Pharmacy, LLC and Alliance Pharma Solutions, LLC
On
January 20, 2023, the Company entered into Membership Interest Purchase Agreements to sell 100% of the outstanding membership interests
of the Company’s former subsidiaries, Community Specialty Pharmacy, LLC (“CSP”) and Alliance Pharma Solutions, LLC
(“APS” d.b.a DelivMeds). The Company also agreed to enter into a Master Service Agreement to operate the businesses prior
to closing. The transactions contemplated by the Membership Interest Purchase Agreements closed on August 22, 2023.
Softell
Inc.
On
February 16, 2024, the Company, together with Softell, and Micro Merchant Systems, Inc. (“MMS”) entered into an asset purchase
agreement (the “MMS APA”) under which MMS agreed to purchase for cash substantially all of the assets of Softell. On February
16, 2024, the parties consummated the closing of the transactions contemplated by the MMS APA. Softell operated a web-based market platform
designed to enable trading among healthcare buyers and sellers of pharmaceuticals, accessories and services.
Superlatus
Inc.
On
March 5, 2024, the Company entered in a Stock Purchase Agreement (“Superlatus SPA”) with Superlatus Foods Inc. (the “Buyer”).
Pursuant to the Superlatus SPA, the Company sold all of the issued and outstanding stock of Superlatus to the Buyer. The $1.00 purchase
price for the Stock was delivered to the Company at the closing, which occurred simultaneously with the execution of the Superlatus SPA.
As a result of the transaction Superlatus is no longer a subsidiary of the Company, and the rights and assets of Superlatus together
with various liabilities and obligations that were specific to Superlatus became rights and obligations of the Buyer.
Historical
Business
We
historically focused on health services IT assets and operations aimed at digitalizing the retail pharmacy experience via an online pharmaceutical
marketplace. Our current primary operations are conducted through our wholly-owned subsidiary, IPS, which is a licensed pharmaceutical
wholesaler and sells brand, generic and non-drug products to customers. IPS customers include all healthcare markets including government
organizations, hospitals, clinics and independent pharmacies nationwide.
We
began operations as Trxade Group, Inc., a Nevada corporation (“Trxade Nevada”) in August of 2010 and spent over two years
creating and enhancing our web-based services. The Company changed its name on June 1, 2021, from “Trxade Group, Inc” to
“TRxADE HEALTH, Inc.” Our services provided pricing transparency, purchasing capabilities and other value-added services
on a single platform focused on serving the nation’s approximately 19,397 independent pharmacies with annual purchasing power of
$67.1 billion (according to the National Community of Pharmacists Association’s 2021 Digest). Our national wholesale supply partners
and manufacturers were able to fulfill orders on our platform in real-time and provide pharmacies and wholesale suppliers with cost-saving
payment terms and next-day delivery capabilities in unrestrictive states. We have expanded significantly since 2015 and served approximately
14,400+ registered members on our sales platform.
Trxade.com
previously operated the Company’s web-based pharmaceutical marketplace engaged in promoting and enabling commerce among independent
pharmacies, small chains, hospitals, clinics, and alternate dispensing sites with large pharmaceutical suppliers nationally. That marketplace
had over 60 national and regional pharmaceutical suppliers providing over 120,000 branded and generic drugs, including over-the-counter
drugs (OTCs), and drugs available for purchase by pharmacists. We served approximately 14,400+ registered members, providing access to
Trxade’s proprietary pharmaceutical database and data analytics regarding medication pricing. We generated revenue from these services
by charging a transaction fee to the seller of the products for sales conducted via the Trxade platform. The buyers did not bear the
cost of transaction fees for the purchases that they made, nor did they pay a fee to join or register with our platform. In February
2024 we divested substantially all of our assets related to our web-based pharmaceutical marketplace previously operated through Softell.
Substantially all of our revenues during Fiscal 2023, Fiscal 2022, and Fiscal 2021 were from platform revenue generated on www.rx.trxade.com,
product sales through IPS, and prescription sales through Community Specialty Pharmacy, LLC.
We
previously had a number of products and services focused on the US market in operation and business assets, which are described below.
Integra
Pharma Solutions, LLC. IPS is intended to serve as our logistics company for pharmaceutical distribution. We currently distribute
through our manufacturer and strategic distribution partners prescription medication, medical devices and over the counter medication
to over 1,600 pharmacies and medical clinics across 38 states.
Trxade
Prime. Trxade Prime previously allowed pharmacy members on the Trxade platform to process, consolidate and ship purchase orders that
were placed directly with Trxade suppliers via Trxade Prime. This service was provided at no cost, with the goal of offering a single
tool with one low order minimum, one invoice, one package and one delivery from multiple quality wholesalers and distributors. Revenue
had been generated from this service through our IPS subsidiary, which provided the consolidation of the orders.
Bonum
Health Application. The “Bonum Health app,” previously provided an overall healthcare experience comparable to a primary
care practitioner, and an online portal as a personal electronic medical record and scheduling system was available on a subscription
basis, primarily as a stand-alone telehealth software application that could be licensed on a business-to-business (B2B) model to clients
as an employment health benefit for the clients’ employees. Revenue was generated from this service through our Bonum Health subsidiary.
Bonum+
Business to Business (B2B). Bonum+ previously bundled telehealth, a COVID-19 risk assessment tool and a Personal Protective Equipment
(PPE) purchasing tool, through a secure mobile dashboard for corporate clients. The B2B platform eased pressure on employees who were
required to report any relevant health issues daily, centralizing communication and contact tracing to deliver risk scores. This allowed
employers to monitor employee COVID-19 risk profiles and streamlined the ordering of new PPE as needed. An integrated artificial intelligence
(AI) tool offered health recommendations and connects employees with board certified physicians, as needed. No revenue was generated
from this product.
SOSRx,
LLC. On February 15, 2022, the Company entered into a relationship with Exchange Health, LLC (“Exchange Health”), a technology
company providing an online platform for manufacturers and suppliers to sell and purchase pharmaceuticals. SOSRx, LLC (“SOSRx”)
was formed, which was owned 51% by the Company and 49% by Exchange Health. SOSRx did not generate material revenue and in February of
2023, the Company voluntarily withdrew from the joint venture agreement.
Superlatus.
As of December 31, 2023, Superlatus was a wholly owned subsidiary of the Company as a result of a merger transaction that closed
in July 2023. Superlatus is a diversified food technology company with distribution capabilities and systems to optimize food security
and population health via innovative Consumer Packaged Goods products, agritech, foodtech, plant-based proteins and alt-protein and includes
wholly-owned subsidiary, Sapientia, Inc., a food tech business. Subsequent to December 31, 2023, the Company divested its entire interest
in Superlatus.
Current
Business – Scienture LLC
Overview
Scienture LLC was originally incorporated in Delaware and commenced operations in 2019. In connection with our acquisition in July 2024,
Scienture became a wholly owned subsidiary of the Company. Scienture’s principal executive offices are located in Commack, New York.
Scienture
LLC is a specialty pharmaceutical company focused on developing and commercializing products for the treatment of CNS and CVS
diseases. Scienture LLC is developing a broad range of novel product candidates including new potential treatments for hypertension,
migraine, pain and thrombosis and other related disorders.
Scienture
LLC’s Strategy
Scienture
LLC’s mission is to improve the lives of patients suffering from CNS and CVS diseases. Scienture LLC’s vision is to be a
leader in the industry by developing and commercializing new medicines for the treatment of CNS and CVS diseases. Key elements of
Scienture LLC’s strategy to achieve this vision include:
| |
● |
Advance
product candidates through clinical studies and toward commercialization. Scienture LLC is in various stages of clinical
development for the product candidates in its pipeline, and it intends to move these programs efficiently toward being commercially
available to patients, subject to approval by the U.S. Food and Drug Administration (the “FDA”). Scienture LLC is
working to obtain regulatory approval of its first product candidate, SCN-102. |
| |
● |
Drive
growth and profitability. Using dedicated sales and marketing resources in the U.S., which Scienture LLC is in the process of
building, Scienture LLC will seek to drive the revenue growth of its product candidates approved for marketing by the
FDA. |
| |
|
|
| |
● |
Continue
to grow pipeline. Scienture LLC will continue to evaluate and seek to develop additional product candidates that it believes
have significant commercial potential through Scienture’s internal research and development efforts. |
| |
|
|
| |
● |
Target
strategic business development opportunities. Scienture LLC is exploring a broad range of strategic opportunities. This may
include in-licensing products and entering into co-promotion and co-development partnerships for Scienture’s product
candidates, although no agreements have been reached. |
Research
and Development and Product Portfolio
Scienture
LLC is committed to the development of innovative product candidates in the CNS and CVS therapeutic areas. The process by which
Scienture LLC intends to bring its product candidates to market and the anticipated launch dates of its product candidates is
depicted in the following table:
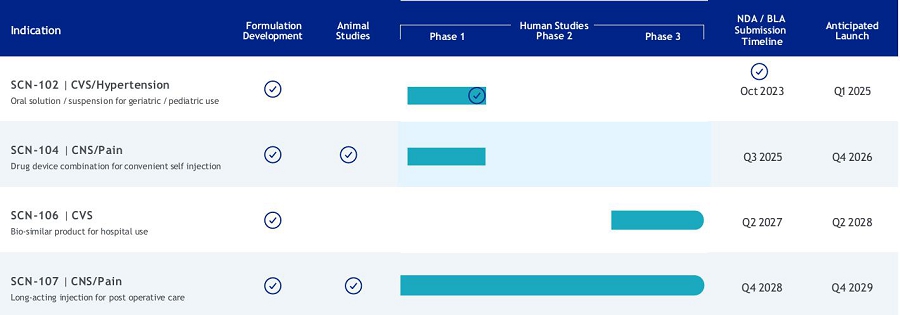
Scienture LLC
does not have any product candidates approved for sale and has not generated any revenue from product sales. Scienture LLC
will not generate revenue from product sales unless and until it successfully obtains regulatory approval for its product
candidates. Scienture LLC is engaged in a
variety of research and development efforts including development of a pipeline of novel product candidates for the treatment of
various disease conditions. Scienture LLC has devoted and will continue to devote significant resources to research and development
activities, and expects to incur significant expenses as Scienture LLC continues advancing its product candidates towards FDA
approval and expanding product indications for approved products and its intellectual property portfolio. Scienture LLC’s
expectations regarding its research and development programs are subject to risks, including the risk that Scienture LLC’s
financial condition and results of operations for fiscal year 2024 and beyond may be materially and adversely affected by delays and
failures in the completion of clinical development of its product candidates, which could increase its costs or delay or limit our
ability to generate revenues.
SCN-102
(ARBLITM - Losartan Oral Suspension)
SCN-102
is an oral liquid formulation of losartan potassium in development under the 505(b)(2) pathway, for (i) treatment of hypertension, to
lower blood pressure in adults and children greater than 6 years old, (ii) reduction of the risk of stroke in patients with hypertension
and left ventricular hypertrophy, and (iii) treatment of diabetic nephropathy with an elevated serum creatinine and proteinuria in patients
with type 2 diabetes and a history of hypertension. Currently, there are no FDA-approved liquid formulations of losartan potassium.
A
Phase I PK study has shown that SCN-102 has close comparability to the immediate-release tablet as depicted in the data below:
| Summary of Statistical Results
for Losartan Potassium Oral Liquid 10 mg/ml (T) versus Losartan Potassium Immediate Release Tablets 100 mg (R) – For Losartan |
| | |
| | |
Geometric Means of treatment: | | |
| | |
| | |
| | |
| | |
| |
| PK Parameter | |
N | | |
Test (T) | | |
Reference (R) | | |
Ratio (%) | | |
Intra-subject %CV | | |
90% CI of Ratio | | |
SABE Result – Bound | | |
SABE SWR | |
| Log
Cmax (ng/ml) | |
| 44 | | |
| 1317.6955 | | |
| 974.6741 | | |
| 135.19 | | |
| 39.6 | | |
| 122.19
– 149.58 | | |
| 0.070 | | |
| 0.3361 | |
| LogAUC0-t
(ng.hr/ml) | |
| 44 | | |
| 1590.6271 | | |
| 1581.0602 | | |
| 100.61 | | |
| 13.2 | | |
| 97.25
– 104.08 | | |
| -0.014 | | |
| 0.1576 | |
| LogAUC0-inf
(ng.hr/ml) | |
| 44 | | |
| 1615.4717 | | |
| 1605.3052 | | |
| 100.63 | | |
| 13.0 | | |
| 97.34
– 104.03 | | |
| -0.014 | | |
| 0.1549 | |
| Summary of Statistical Results
for Losartan Potassium Oral Liquid 10 mg/ml (T) versus Losartan Potassium Immediate Release Tablets 100 mg (R) – For Carboxylic
Acid Metabolite |
| | |
| | |
Geometric Means of treatment: | | |
| | |
| | |
| | |
| | |
| |
| PK Parameter | |
N | | |
Test (T) | | |
Reference (R) | | |
Ratio (%) | | |
Intra-subject %CV | | |
90% CI of Ratio | | |
SABE Result – Bound | | |
SABE SWR | |
| Log
Cmax (ng/ml) | |
| 44 | | |
| 1160.0978 | | |
| 1056.3253 | | |
| 109.82 | | |
| 25.5 | | |
| 102.91
– 117.20 | | |
| -0.028 | | |
| 0.2828 | |
| LogAUC0-t
(ng.hr/ml) | |
| 44 | | |
| 6775.8841 | | |
| 6726.8952 | | |
| 100.73 | | |
| 10.3 | | |
| 98.11
– 103.42 | | |
| -0.006 | | |
| 0.1099 | |
| LogAUC0-inf
(ng.hr/ml) | |
| 44 | | |
| 6872.6739 | | |
| 6823.3736 | | |
| 100.72 | | |
| 10.2 | | |
| 98.13
– 103.39 | | |
| -0.006 | | |
| 0.1071 | |
Specifically,
the Phase I PK study showed that SCN-102 was comparable to immediate release tablets based on the following:
| |
● |
The
overall exposure for the Carboxylic Acid metabolite (EXP-3174) meet the confidence interval 80-125% range. |
| |
|
|
| |
● |
The
overall exposure for Losartan were within the 90% confidence interval. |
| |
|
|
| |
● |
The
Cmax for Losartan analyte, a pro-drug, for SCN-102 was slightly higher than the immediate release tablets (122.19 - 149.58%). This
is due to the fact that the SCN-102 (oral liquid) and the reference product are two different dosage forms. SCN-102 is an oral liquid
formulation and therefore it is expected to have an earlier Cmax than the immediate release tablet. Based on the discussion above,
we believe that the impact of this Cmax difference will be minimal. |
| |
|
|
| |
● |
The
data obtained in the study is similar to the PK study data for the immediate release tablet. |
If
approved, SCN-102 would be the first FDA approved oral liquid formulation of losartan on the market.
Scienture
LLC submitted an Investigational New Drug (“IND”) application to the FDA in September 2022. Multiple human
pharmacokinetics studies were performed, showing close comparability with the oral solid dosage form. In October 2023, Scienture LLC
submitted an NDA for losartan potassium oral suspension to the FDA. In December 2023, the FDA accepted the NDA for review and
assigned a Prescription Drug User Fee Act (“PDUFA”) target action date of August 19, 2024. Despite responding during the
FDA’s review to information requests related to chemistry, manufacturing, and controls (“CMC”), pharmacovigilance,
clinical, microbiology and labeling, the FDA issued a Complete Response Letter to Scienture focused on the CMC information
submitted. Scienture is working expeditiously to prepare the requested information to resubmit the NDA as a Class 1 resubmission,
which carries a two (2) month review and action period following FDA’s receipt.
SCN-104
(Multi-dose Dihydroergotamine Mesylate (“DHE”) injection pen)
The
SCN-104 injection pen is a disposable, multiple fixed dose, single entity combination product comprised of a small molecule drug, SCN-104,
which is administered using a customized injection pen. SCN-104 is a drug product containing DHE as the active ingredient. The mechanism
of action of SCN-104 is mediated through DHE and is exactly the same as that of DHE. DHE is available in the market as a single dose
nasal spray, which has a high degree of variability in clinical outcomes. DHE is also available in the market as single dose ampoules
for injection, however, Scienture believes that the process of dose withdrawal from the ampoule followed by self-injection at the time
of intense need is cumbersome and difficult for the patient.
Scienture
believes that the SCN-104 multi-dose self-injection pen is easy to use and provides enhanced patient convenience. Furthermore, Scienture
believes that the SCN-104 injection pen provides for consistent and accurate delivery of every dose which results in better exposure
compared to the nasal spray formulation. The SCN-104 injection pen is being developed via the 505(b)(2) regulatory pathway. The SCN-104
injection pen is in development for the acute treatment of migraine headaches with or without aura and the acute treatment of cluster
headache episodes.
As
shown in third party studies of DHE, SCN-104’s mechanism of action for its antimigraine effect is due to its potential action as
an agonist at the serotonin 5-HT1D receptors. SCN-104 is intended for subcutaneous administration. SCN-104 is also intended for acute
use and is not intended for chronic administration.
Scienture
has conducted two preclinical studies of SCN-104 and the SCN-104 injection pen: (i) a 30-day repeated dose toxicity study of dimethyl
sulfoxide and caffeine following thrice daily, 3 times per week subcutaneous administration in Sprague-Dawley rats and (ii) a 30-day
repeated dose toxicity study of dimethyl sulfoxide and caffeine following thrice daily, 3 times per week subcutaneous administration
in Göttingen minipigs. The objective of each study was to evaluate the safety and tolerability of the test items with and without
DHE to the subject animals, providing information on important potential toxic effects, target organs, progressive toxic effects, characterization
of a possible dose-response relationship, and an estimate the No-Observed-Adverse-Effect Level (the “NOAEL”). Both studies
were designed for the qualification of the excipients. The animals treated either with DHE + DMSO + caffeine or DMSO + Caffeine formulations
did not reveal any changes attributable to treatment at the end of the treatment/recovery periods. As such, both studies support a conclusion
that SCN-102 is considered to have no toxicological significance across the following attributes – Hematology, Coagulation Parameters,
Clinical Chemistry and Urinalysis.
Scienture
believes the SCN-104 injection pen may offer a significant improvement, in terms of usability and patient acceptability, to the current
standard of care in the market (ampoules for injection). The intended pen delivery system was designed with patients in mind to carry
multiple doses, have a lower volume of injection, and utilize shielded needles to avoid unnecessary exposure.
Scienture
has had initial discussions with the FDA to align on a path forward for this development program. As a result of these discussions, Scienture
learned that its proposed plan for manufacturing NDA registration batches and that the reference product and dose selection of the reference
product that Scienture selected for a comparative regulatory study are acceptable. Scienture also received guidance from the FDA on nonclinical
safety studies and stability testing. The formulation has been scaled up to enable future commercial scale production and the pen has
been optimized for commercial use. As shown below, several pharmacokinetics studies have shown comparability between SCN-104 and the
currently available marketed injection product.
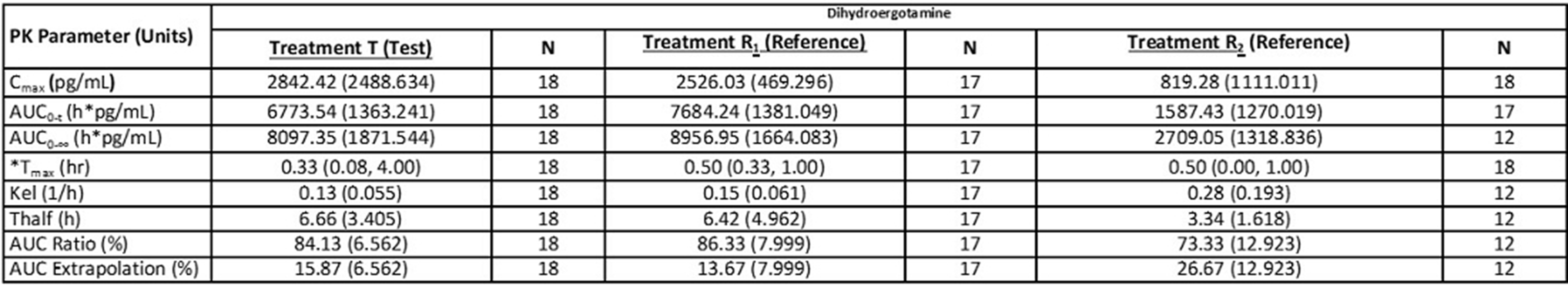
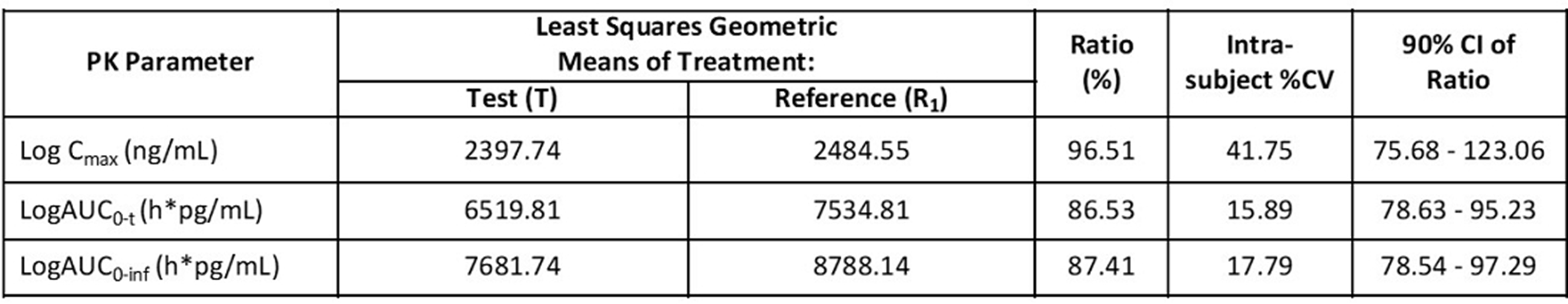
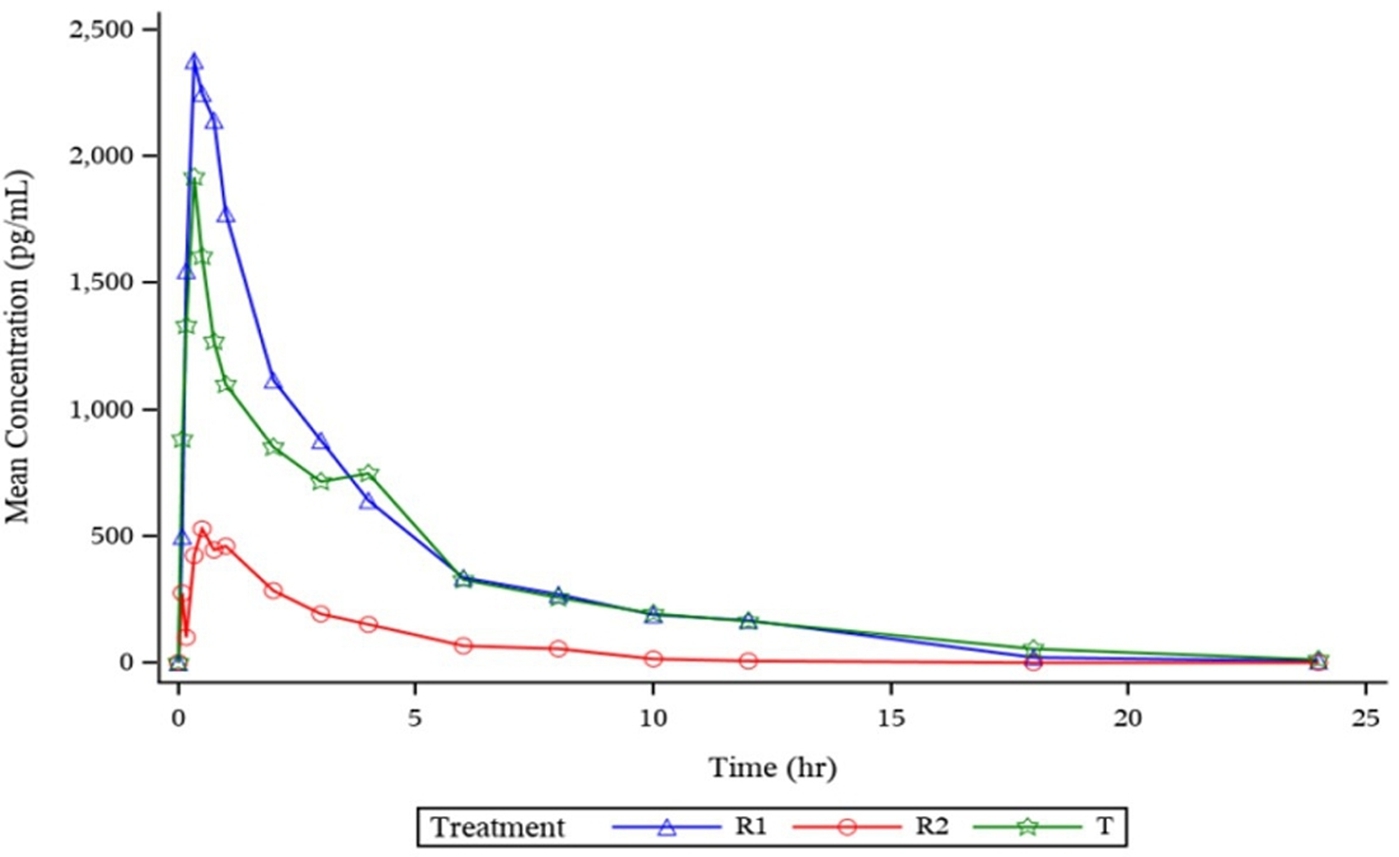
Scienture
is initiating manufacturing activities and planning to conduct bioequivalence studies. Scienture plans to initiate a Phase 1 single dose
study in healthy adults in 2025, following submission of an IND, if the IND is cleared by the FDA.
SCN-106
(Potential Biosimilar)
Scienture
is developing a potential biosimilar, SCN-106, based on Cathflo Activase, a reference product that is a thrombolytic agent that
binds to fibrin in clots and converts entrapped plasminogen to plasmin. SCN-106 is a sterile, purified glycoprotein that is
synthesized using the complementary DNA for natural human tPA obtained from a Chinese hamster ovary cell-line.
Scienture
is working with Anthem Biosciences Pvt, Ltd. to develop a biosimilar product that utilizes the same mechanism(s) of action for the proposed
condition of use, and has the same route of administration, dosage form, and strength as the reference product.
The
CMC development program is focused on establishing the analytical similarity of SCN-106 to the reference product. Multiple clones of
CHO cells have been produced to synthesize lots of SCN-106 which were screened for similarity to the reference product for several key
biochemical quality attributes as well as overall protein yield and finalization of a lead clone.
Scienture
LLC completed a Biosimilar Initial Advisory meeting with the FDA in June 2023 to discuss the CMC, non-clinical, and clinical studies
required for regulatory approval. As a result of this meeting, Scienture learned that its analytical strategy for initiating
analytical similarity studies between SCN-106 and a proposed biosimilar product is acceptable. Scienture also learned that SCN-106
is suitable for further development and received guidance from the FDA on a comparable clinical study needed to demonstrate
biosimilarity of SCN-106 and the reference product.
SCN-107
(Bupivacaine Long-Acting Injection)
SCN-107
is a long-acting injection suspension formulation of a non-opioid analgesic that is indicated for postsurgical local and regional analgesia.
Scienture’s long-acting
formulation, SCN-107, is a novel microsphere-based formulation of bupivacaine that comprises the drug in polymer-based microspheres and
is intended to provide pain management over a period of 5-7 days. The product candidate is designed to potentially provide longer term
post-surgical pain relief compared to the currently available products in the market.
Based
on initial discussions with FDA regarding this program, Scienture believes this product candidate would require at least one Phase 3
clinical trial to support submission of a marketing application.
Scienture
anticipates submitting an IND and, if cleared by the FDA, initiating a Phase 1 single dose study in healthy adults in 2025 to conduct
an initial assessment of safety and tolerability of SCN-107.
Sales
and Marketing
Scienture
intends to market its products through its own sales forces in the U.S. and seek strategic collaborations with other pharmaceutical companies
to commercialize its products outside of the U.S. Scienture is in the process of building a commercial sales and marketing operation
in the U.S., through a partnership with a Contract Sales Organization, to support sales of Scienture’s
products. Once approved, this sales and marketing
organization will include a combination of field teams, virtual sales representatives and omnichannel marketing to effectively reach
Health Care Providers (“HCPs”)
and offer patient education. Scienture’s promotional efforts are expected to further include developing a market access strategy
to obtain commercial and government payor coverage for its products. In addition, Scienture intends to partner with a third-party logistics
provider (“3PL”) and have internal
sales operations and analytics teams to provide state-of-the-art distribution capabilities to wholesalers, pharmacies, institutional
buying groups and hospitals. Scienture believes its commercial operations infrastructure, once
established, will enable it to effectively target healthcare providers to support and grow its products subsequent to market entry.
Customers
The
majority of Scienture’s product sales, if its products are approved by the FDA, are expected to be to pharmaceutical wholesalers,
specialty pharmacies, and distributors who, in turn, would sell such products to pharmacies, hospitals, long term care institutions and
other customers, potentially including federal and state entities.
Market
and Competition
Scienture
is engaged in segments of the pharmaceutical
industry that are highly competitive and rapidly changing. Many large pharmaceutical and biotechnology companies, academic institutions,
governmental agencies, and other public and private research organizations are commercializing or pursuing the development of products
utilizing the same molecules or compounds or for the same indications that Scienture is currently pursuing or may target in the future.
Hypertension
Hypertension
(high blood pressure) is a CVS condition, when the pressure in the blood vessels is too high (140/90 mmHg or higher). According to the
Centers for Disease Control, hypertension, or high blood pressure, affects nearly half of adults in the United States, or 119.9 million people. Hypertension
is defined as a systolic blood pressure of 140 mmHg or higher, and diastolic blood pressure of 90 mmHg or higher. Hypertension is a risk
factor for stroke and heart disease, which are leading causes of death in the U.S. Factors that increase the risk of having high blood
pressure include: older age, genetics, being overweight or obese, not being physically active, high-salt diet and drinking too much alcohol.
Hypertension is clinically diagnosed if, when blood pressure is measured on two different days, the systolic blood pressure readings
on both days is ≥140 mmHg and/or the diastolic blood pressure readings on both days is ≥ 90 mmHg.
The
hypertension market has increased with the commercial launch of several branded products in recent years, as well as the launch of generic
versions of branded drugs, such as Prinvil, Lotensin, Cozaar, Cardizem, Apresoline, Nitrostat and Toprol-XL. Treatment options for hypertension
in the U.S. market can be broadly classified across the following product classes, Angiotensin-converting enzyme (ACE) inhibitors, Angiotensin
II receptor blockers (ARBs), Beta-Blockers, Diuretics and Calcium Channel Blockers.
Scienture’s
product candidate SCN-102, ARBLITM (Losartan Oral Suspension 10mg/mL), is a ready to use oral suspension of losartan for increased
patient convenience and ease of dosing. Losartan is classified as an ARB for treating hypertension and is one of the highest prescribed
molecules for this indication. Current products in the market containing losartan are available only as oral solids, which can be further
compounded to a liquid formulation. Scienture believes that ARBLITM, if approved by the FDA, would be the first liquid formulation
of losartan on the market that does not require compounding and has reduced dosing volume and long-term shelf life at room temperature
storage.
Migraine
Migraine
is a painful, complex neurological disorder consisting of recurring painful attacks that can significantly impact quality of life. Migraine
headaches are often characterized by throbbing pain, extreme sensitivity to light or sound, and potentially nausea and vomiting. The
World Health Organization categorizes migraine as one of the most disabling medical illnesses worldwide. The American Research Foundation
categorizes migraine as the third most prevalent illness in the world, and nearly 1 in 4 U.S. households includes someone with migraines.
Migraine is estimated to affect over 39 million individuals in the U.S.
Current
products in the market that are available to treat migraine headaches, include CGRP antagonists (calcitonin gene related peptide), which
is a class of products first introduced in 2018 (Nurtec, Ubrelvy), Botox, branded and generic versions of triptans (Imitrex, Maxalt,
Relpax), and ergot alkaloids (Ergotamine and Dihydroergotamine (DHE)).
Scienture’s
product candidate, SCN-104, is supplied in a multi-dose pen-based delivery system for self-injection and increased patient convenience.
The product candidate is in development for the acute treatment of migraine headaches with or without aura and the acute treatment of
cluster headache episodes.
Thrombotically
Occluded Catheter (CVAD) Management
Catheters,
which are a type of a Central Venous Access Device (“CVAD”), are employed to deliver life-sustaining therapies. They can
be used for short-term or long-term infusion of antibiotics, parenteral nutrition, chemotherapy, blood and blood products in patients
with limited peripheral access. More than 7 million CVADs are inserted each year in patients in the United States. Occlusion of catheters
while in use can complicate patient care by interrupting the administration of medications and solutions, delaying or disrupting therapies
and leading to additional procedures such as catheter replacement. Occlusion is the most common noninfectious complication in the long-term
use of CVADs and may occur soon after insertion of a device or develop at any time. About 58% of catheter occlusions are thrombotic,
resulting from the formation of a thrombus within, surrounding, or at the tip of the catheter.
Scienture’s
product candidate, SCN-106, is a thrombolytic agent currently in development. Scienture plans to develop SCN-106 through the FDA’s
351(k) pathway for biosimilars.
Postoperative
Pain
Post-surgery
pain, also known as postoperative pain, is pain that a patient experiences after a surgical procedure. Pain can be caused by a number
of factors, including: the type of procedure, the size of the operation, and medications used during surgery. Chronic pain can negatively
impact a patient’s rehabilitation, quality of life, and the results of the procedure.
Current
drug product treatments available in the market for treating postoperative pain include IV and oral opioids, injectable local anesthetics,
and steroidal and non-steroidal analgesics. Marketed products include branded and generic versions of Celebrex, Ketalar, Exparel, Lyrica,
Neurontin and Astromorph.
Scienture’s
product candidate, SCN-107, is a microsphere based long-acting injection of Bupivacaine, a local anesthetic, in development for postsurgical
analgesia. SCN-107 is designed to be a non-opioid treatment regimen with rapid onset of action and analgesia that is intended to provide
coverage over a period of 5-7 days.
Manufacturing
Scienture
currently depends on third-party commercial manufacturing organizations (“CMOs”) for all manufacturing operations, including
the production of raw materials, finished dosage form product, and product packaging for both its planned commercial scale manufacturer
and the products used in its preclinical and clinical research. Scienture does not own or operate manufacturing facilities for the production
of any of its product candidates nor does Scienture have plans to develop its own manufacturing operations in the foreseeable future
to support clinical trials or commercial production. Scienture currently employs internal resources to manage its manufacturing contractors.
Scienture
is in discussion with CMOs headquartered in North America, Europe and Asia for its pipeline product candidates. These CMOs offer a comprehensive
range of commercial contract manufacturing and packaging services.
If
Scienture fails to produce its products and product candidates in the volumes that Scienture requires on a timely basis, or fails to
comply with stringent regulations applicable to pharmaceutical drug manufacturers, Scienture may face delays in the development and commercialization
of its products and product candidates or be required to withdraw its products from the market for
risks associated with manufacturing and supply of its products and product candidates.
License
Agreements
On
May 26, 2020, Scienture LLC entered into Feasibility Study and Animal Trial Material Manufacturing Agreement with Innocore Technologies,
B.V. (“Innocore”), as amended on December 2, 2022 (the “Innocore License”), for certain intellectual property
rights. Under the Innocore License, Innocore granted Scienture a worldwide exclusive, milestone, royalty-bearing and sublicensable license
to certain patent rights for the research and development of SCN-107 in postsurgical local and regional analgesia. Pursuant to the Innocore
License, Scienture is required to make low single-digit percentage royalty payments based on annual net sales of licensed products for
the first three years of sales on a country-by-country basis, subject to a low single digit increase as of the fourth year of sales on
a country-by-country basis. Scienture is required to remunerate Innocore for the development of the licensed product, subject to a limit
of $0.4 million for certain safety and toxicity studies which will be deducted from certain development and regulatory milestones as
described below. Scienture is required to make development and regulatory milestone payments up to €2.7 million in the aggregate,
commercial sale milestone payments of up to €18.875 million in the aggregate, and maintenance fees of €0.25 million annually,
subsequent to the first regulatory filing, until the date that Scienture begins making royalty payments based on annual net sales, up
to €0.5 million of which may be credited toward the regulatory milestone payments. As of October 25, 2024, the Company had made
aggregate payments to Innocore of $1,021,089.37 in connection with the Innocore License.
The
Innocore License is terminable by either the Company or Innocore on thirty (30) days’ prior written notice if the terminating party
determines in good faith, that it is technically or legally not feasible, or commercially not viable to jointly develop a formulation
which meets the specifications described in the Innocore License. The Innocore License can also be terminated for any material breach
of the Innocore License that remains uncured after thirty (30) days and if either party files for insolvency under any applicable foreign,
federal or state law.
Intellectual
Property
Overview
Scienture
continues to build its intellectual property portfolio to provide protection for its technologies,
products, and product candidates. Scienture seeks patent protection, where appropriate, both in the U.S. and internationally for
products and product candidates.
Scienture’s
intended objective is to protect its innovations and proprietary products by, among other things, filing patent applications in the U.S.
and abroad, including Europe, Canada, and other countries when appropriate. Scienture also relies on trade secrets, know-how, proprietary
knowledge, continuing technological innovation, and in-licensing opportunities to develop and maintain its proprietary position. Scienture
cannot be sure that patents will be granted with respect to its pending patent applications or with respect to any patent applications
filed by it in the future, nor can Scienture be sure that any of its existing patents or any patents that may be granted to it in the
future will be commercially useful in protecting its technology or its products. Scienture cannot be sure that any patents, if granted,
will sustain a legal challenge.
Patent
Portfolio
SCN-102
SCN-102
will soon have two orange book listable formulation composition and method of use patents in the U.S. One of them is already issued (Patent
#: 11,890,273, Issue Date: February 6, 2024, titled “LOSARTAN LIQUID FORMULATIONS AND METHODS OF USE”, Expiration Date: October
7, 2041) and the other patent application is allowed, with the issue fee paid on July 24, 2024 (Appl. No. 18/421,405; Filing Date: January
24, 2024, titled “LOSARTAN LIQUID FORMULATIONS AND METHODS OF USE”). A third application is pending (Appl. No. 18/061,819;
Filing Date: December 5, 2022; Expiration: on or after October 7, 2041).
SCN-104
SCN-104
has a formulation composition and method of use application pending in the U.S. (Appl. No. 17/757,924; Filing Date: June 23, 2022; Expiration Date: June 15, 2035).
SCN-106
SCN-106
is a potential biosimilar and considered by the Company to be part of its product development portfolio, however the Company is not pursuing
patent protection for this product.
SCN-107
SCN-107
has a formulation composition and method of use application pending in the U.S. (Appl. No. 17/996,995; Filing Date: October 24, 2022; Expiration Date: on or after April 22, 2041).
Applications in Canada and Europe are currently pending. As described above, the Company licenses certain patent rights from Innocore
for the research and development of SCN-107.
Collaborations
and Licensing Arrangements
Scienture
LLC entered into exclusive license and commercial agreements on August 28, 2022 and April 24, 2023, with Kesin Pharma Corporation (“Kesin”),
a related party, pursuant to which Scienture granted the exclusive license rights to commercialize SCN-102 and SCN-104, respectively
to Kesin for use in the United States of America (together, the “Kesin Agreement”). In consideration of the rights granted,
Scienture received milestone payments and reimbursement of costs actually incurred related to SCN-102 and SCN-104.
On
March 13, 2024, the parties terminated the Kesin Agreement by entering a Confidential Termination Agreement (the “Kesin Termination
Agreement”), and the parties agreed that Scienture LLC would pay Kesin a total gross amount of $1.285 million upon commercialization
of either SCN-102 or SCN-104 via a royalty arrangement. The Kesin Termination Agreement also requires that if the full $1.285 million
has not been repaid within two years of the earlier of (i) commercial launch of a product or (ii) 120 days after FDA approval of a product,
then interest will accrue prospectively at a rate of 8% annually on the unpaid balance.
In
August 2024, Kesin demanded immediate payment of the full amount under the Kesin Termination Agreement, alleging the full amount is payable
in connection with the consummation Scienture’s business combination with the Company. Scienture has disputed that the amount is
now payable, and the parties are in discussions to resolve the issue. There can be no assurance that an amicable resolution will be obtained.
If Kesin brings a legal action, Scienture will vigorously defend it.
Government
Regulation
U.S.
Drug Development Process
In
the United States, pharmaceutical products are subject to extensive regulation by the FDA. The Federal Food, Drug, and Cosmetic Act (the
“FDCA”) and other federal and state statutes and regulations, govern, among other things, the research, development, testing,
manufacture, storage, recordkeeping, approval, labeling, promotion and marketing, distribution, post-approval monitoring and reporting,
sampling, and import and export of pharmaceutical products. Scienture, along with third-party contractors, will be required to navigate
the various preclinical, clinical and commercial approval requirements of the governing regulatory authorities of the countries in which
Scienture wishes to conduct studies or seek approval of its product candidates. Failure to comply with applicable United States requirements
may subject a company to a variety of administrative or judicial sanctions, such as FDA refusal to approve pending applications, withdrawal
of an approval, warning or untitled letters, clinical holds, product recalls or withdrawals from the market, product seizures, total
or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement
of profits, civil penalties, and criminal prosecution.
FDA
approval is required before any new unapproved product or a product with certain changes to a previously approved product, including
a new use of a previously approved drug, can be marketed in the United States. The steps required to be completed by the FDA before a
drug may be marketed in the United States generally include the following:
| |
● |
completion
of preclinical laboratory tests, animal studies, and formulation studies performed in accordance with the FDA’s Good Laboratory
Practice (“GLP”) regulations; |
| |
|
|
| |
● |
submission
to the FDA of an IND application for human clinical testing, which must become effective before human clinical trials may begin and
must be updated annually or when significant changes are made; |
| |
|
|
| |
● |
approval
by an independent institutional review board (“IRB”) or ethics committee at each clinical site before the clinical trial
is commenced; |
| |
|
|
| |
● |
performance
of adequate and well-controlled human clinical trials in accordance with applicable IND regulations, good clinical practices (“GCPs”)
requirements and other clinical-trial related regulations to establish the safety and efficacy of the proposed drug for each indication; |
| |
|
|
| |
● |
preparation
and submission to the FDA of an NDA or biologics license application (“BLA”), after completion of all pivotal clinical
trials, which includes not only the results of the clinical trials, but also, detailed information on the chemistry, manufacture
and quality controls for the product candidate and proposed labeling; |
| |
|
|
| |
● |
satisfactory
completion of an FDA Advisory Committee review, if applicable; |
| |
● |
a
determination by the FDA within 60 days of its receipt of an NDA or BLA to file the application for review; |
| |
|
|
| |
● |
satisfactory
completion of an FDA pre-approval inspection of the manufacturing facility or facilities at which the proposed drug is produced to
assess compliance with current good manufacturing practices (“GMPs”) regulations and of selected clinical trial sites
to assess compliance with GCPs; and |
| |
|
|
| |
● |
FDA
review and approval of the NDA or BLA to permit commercial marketing of the product for particular indications for use in the United
States. |
Satisfaction
of FDA pre-market approval requirements typically takes many years and the actual time required may vary substantially based upon the
type, complexity, and novelty of the product or disease.
Preclinical
and Clinical Development
Preclinical
tests include laboratory evaluation of product chemistry, formulation, and toxicity, as well as animal trials to assess the characteristics
and potential safety and efficacy of the product candidate. The conduct of the preclinical tests must comply with federal regulations
and requirements, including GLP. The results of preclinical testing are submitted to the FDA as part of an IND application along with
other information, including information about the product candidate, chemistry, manufacturing and controls, any available human data
or literature to support the use of the product candidate and a proposed clinical trial protocol. Long term preclinical tests, such as
animal tests of reproductive toxicity and carcinogenicity, may continue after the IND is submitted.
An
IND application must become effective before human clinical trials may begin. The IND application automatically becomes effective 30
days after receipt by the FDA, unless the FDA, within the 30-day period, raises safety concerns or questions relating to one or more
proposed clinical trials and places the clinical trial on clinical hold. In such a case, the IND sponsor and the FDA must resolve any
outstanding concerns or questions before the clinical trial can begin. The FDA may also impose clinical holds on a product candidate
at any time before or during clinical trials due to safety concerns, non-compliance or other issues affecting the integrity of the trial.
Accordingly, submission of an IND application may or may not result in the FDA allowing clinical trials to commence and, once begun,
issues may arise that could cause the trial to be suspended or terminated.
Clinical
trials involve the administration of the investigational drug product to human subjects under the supervision of a qualified investigator.
Clinical trials must be conducted: (i) in compliance with federal regulations; (ii) in compliance with GCP, an international standard
meant to protect the rights and health of clinical research participants and to define the roles of clinical trial sponsors, administrators,
and monitors; as well as (iii) under protocols detailing the objectives of the trial, the parameters to be used in monitoring safety,
and the effectiveness criteria to be evaluated. Each protocol involving testing on United States patients and subsequent protocol amendments
must be submitted to the FDA as part of the IND. Furthermore, an independent IRB or ethics committee for each site proposing to conduct
the clinical trial must review and approve the plan for any clinical trial and its informed consent form before the clinical trial begins
at that site, and must monitor the study until completed. An IRB is charged with protecting the welfare and rights of trial participants
and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in
relation to anticipated benefits.
Regulatory
authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects
are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objects. The FDA may order the temporary,
or permanent, discontinuation of a clinical trial at any time, or impose other sanctions, if it believes that the clinical trial is not
being conducted in accordance with FDA requirements. Further, an IRB may also require the clinical trial at the site to be halted, either
temporarily or permanently, for failure to comply with the IRB’s requirements, or may impose other conditions. Some trials also
include oversight by an independent group of qualified experts organized by the clinical trial sponsor, known as a data safety monitoring
board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain
data from the study and may recommend a clinical trial to be halted if it determines that there is an unacceptable safety risk for subjects
or other grounds, such as futility.
Clinical
trials to support an NDA or BLA for marketing approval are typically conducted in three sequential phases, but the phases may overlap
or be combined. In Phase 1 clinical trials, the investigational product is typically introduced into a limited population of healthy
human subjects or patients with the target disease or condition. These trials are designed to test the safety, dosage tolerance, pharmacokinetics
and pharmacological actions of the investigational product, to identify side effects associated with increasing doses, and, if possible,
to gain early evidence on effectiveness. Phase 2 clinical trials usually involve administering the investigational product to a limited
patient population with the specified disease or condition to evaluate the preliminarily efficacy, dosage tolerance, and optimum dosage,
and to identify possible adverse effects and safety risks. Phase 3 clinical trials are typically undertaken in a larger number of patients,
typically at geographically dispersed clinical trial sites, to provide substantial evidence of clinical efficacy and to further test
for safety in an expanded and diverse patient population. These clinical trials are intended to permit the FDA to evaluate the overall
benefit-risk relationship of the investigational product and to provide adequate information for the labeling of the product candidate.
In
reviewing an NDA or BLA, the FDA will consider all information submitted in the application, including the results of all clinical trials
conducted. In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved
to gain more information about the product. These so-called Phase 4 studies may be made a condition to approval of the NDA or BLA. These
trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication and further document
clinical benefit in the case of drugs approved under accelerated approval regulations. Failure to exhibit due diligence with regard to
conducting Phase 4 clinical trials could result in the withdrawal of approval for products.
Concurrent
with clinical trials, companies may complete additional animal studies and develop additional information about the biological characteristics
of the product candidate, and must finalize a process for manufacturing the product in commercial quantities in accordance with current
GMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among
other things, must develop methods for testing the identity, strength, quality and purity of the final product. Additionally, appropriate
packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo
unacceptable deterioration over its shelf life.
During
all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical
data, and clinical study investigators. Progress reports detailing the results of the clinical trials, among other information, must
be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and the investigators for serious
and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the product candidate,
findings from animal or in vitro testing that suggest a significant risk for human subjects, and any clinically important increase in
the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure.
NDA
and BLA Submission and Review
Assuming
successful completion of the required clinical testing in accordance with all applicable regulatory requirements, an NDA or BLA application
which includes, among other information, the results of product development, preclinical studies and clinical trials is submitted to
the FDA. FDA approval of the application is required before marketing of the product may begin in the United States. The application
must include, among other things, the results of all trials and preclinical testing, and other testing and a compilation of data relating
to the product’s pharmacology, chemistry, manufacture, controls and proposed labeling. The cost of preparing and submitting an
NDA or BLA is substantial.
The
FDA has 60 days from its receipt of an NDA or BLA to either issue a Refuse to File Letter or accept the NDA or BLA for filing, indicating
that it is sufficiently complete to permit substantive review. Once the submission is accepted for filing, the FDA begins an in-depth
review. The FDA has agreed to certain performance goals in the review of NDAs and BLAs. Under applications subject to the performance
goals of the PDUFA, the FDA has a goal of responding to standard review NDAs and BLAs within ten months after it accepts the application
for filing, or, if the application qualifies for priority review, six months after the FDA accepts the application for filing, but this
timeframe can be extended, such as by the submission of major amendments by applicants during the review period. The FDA reviews an application
to determine, among other things, whether the product is safe and effective and the facility in which it is manufactured, processed,
packed or held meets standards designed to assure the product’s continued safety, purity and potency.
The
FDA may refer applications for novel drug products, or drug products that present difficult questions of safety or efficacy, to an advisory
committee—typically a panel that includes clinicians and other experts—for review, evaluation, and a recommendation as to
whether the application should be approved. The FDA is not bound by the recommendation of an advisory committee, but it generally follows
such recommendations. Before approving an application, the FDA will typically inspect one or more clinical sites to assure compliance
with GCPs. Additionally, the FDA will inspect the facility or the facilities at which the proposed product is manufactured. If the FDA
determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies
in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional
information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
After
the FDA evaluates the application and conducts inspections of the manufacturing facilities where the investigational product and/or its
drug substance will be produced, it issues either an approval letter or a Complete Response Letter. An approval letter authorizes commercial
marketing of the drug with approved prescribing information for specific indications. A Complete Response Letter indicates that the review
cycle of the application is complete and the application is not ready for approval. A Complete Response Letter generally outlines the
deficiencies in the submission, except that where the FDA determines that the data supporting the application are inadequate to support
approval, the FDA may issue the Complete Response Letter without first conducting required inspections or reviewing proposed labeling.
In issuing the Complete Response Letter, the FDA may require substantial additional clinical data and/or other significant, expensive,
and time-consuming requirements related to clinical trials, preclinical studies and/or manufacturing. If a Complete Response Letter is
issued, the applicant may either resubmit the NDA or BLA, addressing all of the deficiencies identified in the letter, withdraw the application
or request a hearing. The FDA has committed to reviewing resubmissions of the NDA or BLA addressing such deficiencies in two or six months
depending on the type of information included. Even if such data are submitted, however, the FDA may ultimately decide that the NDA or
BLA does not satisfy the criteria for approval.
If
regulatory approval of a product is granted, such approval will be granted for a particular indication(s) and may include limitations
on the indicated use(s) for which such product may be marketed. Further, the FDA may require that certain contraindications, warnings
or precautions be included in the product labeling or may condition the approval of the application on other changes to the proposed
labeling, development of adequate controls and specifications, or a commitment to conduct post-market testing or clinical trials and
surveillance to monitor the effects of approved products. As a condition of NDA or BLA approval, the FDA may require a risk evaluation
and mitigation strategy (“REMS”) to help ensure that the benefits of the drug outweigh the potential risks. REMS can include
medication guides, communication plans for healthcare professionals, and elements to assure safe use (“ETASU”). ETASU can
include, but are not limited to, special training or certification for prescribing or dispensing, dispensing only under certain circumstances,
special monitoring, and the use of patient registries. The requirement for REMS can materially affect the potential market and profitability
of the product. Moreover, product approval may also be conditioned on substantial post-approval testing, such as Phase 4 post-market
studies, and surveillance to monitor the product’s safety or efficacy, and the FDA may limit further marketing of the product based
on the results of these post-approval studies. Once granted, product approvals may be withdrawn if compliance with regulatory standards
is not maintained or problems are identified following initial marketing.
Changes
to some of the conditions established in an approved application, including changes in indications, labeling, or manufacturing processes
or facilities, require submission and FDA approval of a new NDA or BLA, or NDA or BLA supplement before the change can be implemented.
An NDA or BLA supplement for a new indication typically requires clinical data similar to that in the original application, and the FDA
uses the same procedures and actions in reviewing NDA and BLA supplements as it does in reviewing NDAs and BLAs. As with new NDAs and
BLAs, the review process is often significantly extended by requests for additional information or clarification.
505(b)(2)
NDA Approval Process
Section
505(b)(2) of the FDCA provides an alternate regulatory pathway for the FDA to approve a new product and permits reliance for such approval
on published literature or an FDA finding of safety and effectiveness for a previously approved drug product. Specifically, section 505(b)(2)
permits the filing of an NDA where one or more of the investigations relied upon by the applicant for approval were not conducted by
or for the applicant and for which the applicant has not obtained a right of reference. Typically, 505(b)(2) applicants must perform
additional trials to support the change from the previously approved drug and to further demonstrate the new product’s safety and
effectiveness. The FDA may then approve the new product candidate for all or some of the labeled indications for which the referenced
product has been approved, as well as for any new indication sought by the section 505(b)(2) applicant.
Regulation
of Combination Products in the United States
Certain
products may be comprised of components, such as drug components and device components, that would normally be regulated under different
types of regulatory authorities, and frequently by different centers at the FDA. These products are known as combination products. Specifically,
under regulations issued by the FDA, a combination product may be:
| |
● |
a
product comprised of two or more regulated components that are physically, chemically, or otherwise combined or mixed and produced
as a single entity; |
| |
|
|
| |
● |
two
or more separate products packaged together in a single package or as a unit and comprised of drug and device products, device and
biological products, or biological and drug products; |
| |
|
|
| |
● |
a
drug, or device, or biological product packaged separately that according to its investigational plan or proposed labeling is intended
for use only with an approved individually specified drug, or device, or biological product where both are required to achieve the
intended use, indication, or effect and where upon approval of the proposed product the labeling of the approved product would need
to be changed, e.g., to reflect a change in intended use, dosage form, strength, route of administration, or significant change in
dose; or |
| |
|
|
| |
● |
any
investigational drug, or device, or biological product packaged separately that according to its proposed labeling is for use only
with another individually specified investigational drug, device, or biological product where both are required to achieve the intended
use, indication, or effect. |
Under
the FDCA and its implementing regulations, the FDA is charged with assigning a center with primary jurisdiction, or a lead center, for
review of a combination product. The designation of a lead center generally eliminates the need to receive approvals from more than one
FDA component for combination products, although it does not preclude consultations by the lead center with other components of FDA.
The determination of which center will be the lead center is based on the “primary mode of action” of the combination product.
Thus, if the primary mode of action of a drug-device combination product is attributable to the drug product, the FDA center responsible
for premarket review of the drug product would have primary jurisdiction for the combination product. The FDA has also established an
Office of Combination Products to address issues surrounding combination products and provide more certainty to the regulatory review
process. That office serves as a focal point for combination product issues for agency reviewers and industry. It is also responsible
for developing guidance and regulations to clarify the regulation of combination products, and for assignment of the FDA center that
has primary jurisdiction for review of combination products where the jurisdiction is unclear or in dispute.
A
combination product with a drug primary mode of action generally would be reviewed and approved pursuant to the drug approval processes
under the FDCA. In reviewing the NDA application for such a product, however, FDA reviewers in the drug center could consult with their
counterparts in the device center to ensure that the device component of the combination product met applicable requirements regarding
safety, effectiveness, durability and performance. In addition, under FDA regulations, combination products are subject to current GMP
requirements applicable to both drugs and devices, including the Quality System regulations applicable to medical devices.
Post-Approval
Requirements
Once
an NDA or BLA is approved, a product will be subject to pervasive and continuing regulation by the FDA including, among other things,
requirements relating to current GMPs, quality controls, record-keeping, reporting of adverse experiences, periodic reporting, product
sampling and distribution, and advertising and promotion of the product. For instance, the FDA closely regulates the post-approval marketing
and promotion of drugs, including standards and regulations for direct-to-consumer advertising, off-label promotion, industry-sponsored
scientific and educational activities and promotional activities involving the internet. Drugs may be marketed only for the approved
indications and in accordance with the provisions of the approved labeling. Failure to comply with these requirements can result in adverse
publicity, warning letters, corrective advertising, and potential civil and criminal penalties. Physicians may prescribe legally available
products for uses that are not described in the product’s labeling and that differ from those tested by Scienture and approved
by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment
for many patients in varied circumstances. The FDA does not regulate the practice of medicine by physicians or their choice of treatments.
The FDA does, however, regulate manufacturer’s communications on the subject of off-label use of their products.
In
addition, quality control, drug manufacture, packaging, and labeling procedures must continue to conform to current GMPs after approval.
Drug manufacturers and certain of their subcontractors are required to register their establishments with the FDA and certain state agencies,
and are subject to periodic unannounced inspections by the FDA, and certain state agencies for compliance with current GMPs, which impose
certain organizational, procedural and documentation requirements with respect to manufacturing and quality assurance activities. Changes
to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval
before being implemented. FDA regulations also require investigation and correction of any deviations from current GMPs and impose reporting
requirements upon Scienture and any third-party manufacturers that Scienture may decide to use. NDA or BLA holders using contract manufacturers,
laboratories or packagers are responsible for the selection and monitoring of qualified firms, and, in certain circumstances, qualified
suppliers to these firms. Drug manufacturers and other parties involved in the drug supply chain for prescription drug products must
also comply with product tracking and tracing requirements and notify the FDA of counterfeit, diverted, stolen and intentionally adulterated
products or products that are otherwise unfit for distribution in the United States. The discovery of violative conditions, including
failure to conform to current GMPs, could result in enforcement actions that interrupt the operation of any such facilities or the ability
to distribute products manufactured, processed or tested by them. Accordingly, manufacturers must continue to expend time, money, and
effort in the areas of production and quality-control to maintain compliance with current GMPs.
The
FDA may withdraw product approvals or request product recalls if a company fails to comply with regulatory standards or is not maintained,
if problems occur following initial marketing, or if previously unrecognized problems are subsequently discovered. Later discovery of
previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes,
or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition
of post-market studies or clinical trials to assess new safety risks; or imposition of distribution restrictions or other restrictions
under a REMS program. Other potential consequences include, among other things:
| |
● |
restrictions
on the marketing or manufacturing of a product, complete withdrawal of the product from the market or product recalls; |
| |
|
|
| |
● |
fines,
warning letters or holds on post-approval clinical trials; |
| |
● |
refusal
of the FDA to approve pending applications or supplements to approved applications, or suspension or revocation of existing product
approvals; |
| |
|
|
| |
● |
product
seizure or detention, or refusal of the FDA to permit the import or export of products; |
| |
|
|
| |
● |
consent
decrees, corporate integrity agreements, debarment or exclusion from federal healthcare programs; |
| |
|
|
| |
● |
mandated
modification of promotional materials and labeling and the issuance of corrective information; |
| |
● |
the
issuance of safety alerts, Dear Healthcare Provider letters, press releases and other communications containing warnings or other
safety information about the product; or |
| |
|
|
| |
● |
injunctions
or the imposition of civil or criminal penalties. |
U.S.
Patent Term Restoration
Depending
upon the timing, duration and specifics of the potential FDA approval of Scienture’s product candidates, some of its U.S. patents
may be eligible for limited patent term extension. The Hatch-Waxman Amendments permit a patent restoration term, often referred to as
patent term extension, of up to five years as compensation for patent term lost during product development and the FDA regulatory review
process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s
approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission
date of an NDA plus the time between the submission date of an NDA and the approval of that application. Only one patent applicable to
an approved drug is eligible for the extension and the application for the extension must be submitted prior to the expiration of the
patent. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves or denies the application for any patent
term extension or restoration.
U.S.
Marketing Exclusivity
Market
exclusivity provisions under the FDCA can also delay the submission or the approval of certain marketing applications, including 505(b)(2)
applications. The FDA provides three years of marketing exclusivity for an NDA (including a 505(b)(2) application), or supplement to
an existing NDA, if new clinical investigations, other than bioavailability studies, that were conducted or sponsored by the applicant
are deemed by the FDA to be essential to the approval of the application. Three-year exclusivity is typically awarded to innovative changes
to a previously-approved drug product, such as new indications, dosage forms or strengths. This three-year exclusivity covers only the
modification for which the drug received approval on the basis of the new clinical investigations and does not prohibit the FDA from
approving applications for drugs that do not have the innovative change, such as generic copies of the original, unmodified drug product.
Three-year exclusivity blocks approval of 505(b)(2) applications and Abbreviated New Drug Applications (“ANDAs”), but will
not delay the submission or approval of a full NDA. However, an applicant submitting a full NDA would be required to conduct or obtain
a right of reference to all of the nonclinical studies and adequate and well-controlled clinical trials necessary to demonstrate safety
and effectiveness. Orphan drug exclusivity, as described above, may offer a seven-year period of marketing exclusivity, except in certain
circumstances. Pediatric exclusivity is another type of regulatory market exclusivity in the United States. Pediatric exclusivity, if
granted, adds six months to existing exclusivity periods, including exclusivity attaching to certain patent certifications. This six-month
exclusivity, which runs from the end of other exclusivity protection and patent terms, may be granted based on the voluntary completion
of a pediatric trial in accordance with an FDA-issued “Written Request” for such a trial, provided that at the time pediatric
exclusivity is granted there is not less than nine months of term remaining.
Biosimilars
and Exclusivity
The
ACA, which was signed into law in March 2010, included a subtitle called the Biologics Price Competition and Innovation Act of 2009 (the
“BPCIA”). The BPCIA established a regulatory scheme authorizing the FDA to approve biosimilars and interchangeable biosimilars.
A biosimilar is a biological product that is highly similar to an existing FDA-licensed “reference product.” The FDA has
issued multiple guidance documents outlining an approach to review and approval of biosimilars. Under the BPCIA, a manufacturer may submit
an application for licensure of a biologic product that is “biosimilar to” or “interchangeable with” a previously
approved biological product or “reference product.” In order for the FDA to approve a biosimilar product, it must find that
there are no clinically meaningful differences between the reference product and proposed biosimilar product in terms of safety, purity
and potency. For the FDA to approve a biosimilar product as interchangeable with a reference product, the agency must find that the biosimilar
product can be expected to produce the same clinical results as the reference product, and (for products administered multiple times)
that the biologic and the reference biologic may be switched after one has been previously administered without increasing safety risks
or risks of diminished efficacy relative to exclusive use of the reference biologic.
Under
the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date of approval of
the reference product. The FDA may not approve a biosimilar product until 12 years from the date on which the reference product was approved.
Even if a product is considered to be a reference product eligible for exclusivity, another company could market a competing version
of that product if the FDA approves a full BLA for such product containing the sponsor’s own preclinical data and data from adequate
and well-controlled clinical trials to demonstrate the safety, purity and potency of their product. The BPCIA also created certain exclusivity
periods for biosimilars approved as interchangeable products. Since the passage of the BPCIA, many states have passed laws or amendments
to laws, including laws governing pharmacy practices, which are state regulated, to regulate the use of biosimilars.
Orphan
Drug Designation
Under
the Orphan Drug Act, the FDA may grant orphan drug designation to a drug intended to treat a rare disease or condition—generally
a disease or condition with either a patient population that affects fewer than 200,000 individuals in the United States or a patient
population greater than 200,000 individuals in the United States and there is no reasonable expectation that the cost of developing and
making available the drug will be recovered from sales of the drug in the United States. Orphan drug designation must be requested before
submitting an NDA or BLA. After the FDA grants orphan drug designation, the generic identity of the product and its potential orphan
use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory
review and approval process.
The
first applicant to receive FDA approval for a particular active ingredient to treat a particular disease with FDA orphan drug designation
is entitled to a seven-year exclusive marketing period in the United States for that product, for that indication. During the seven-year
exclusivity period, the FDA may not approve any other applications to market the same product for the same disease, except in limited
circumstances, such as a showing of clinical superiority to the product with orphan drug exclusivity or if the FDA finds that the holder
of the orphan drug exclusivity has not shown that it can assure the availability of sufficient quantities of the orphan drug to meet
the needs of the patients with the disease or condition for which the product was designated. Orphan drug exclusivity does not prevent
the FDA from approving a different product for the same disease or condition, or the same product for a different disease or condition.
Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the NDA or BLA application user
fee.
A
designated orphan drug may not receive orphan drug exclusivity if it is approved for a use that is broader than the indication for which
it received orphan drug designation. In addition, orphan drug exclusive marketing rights in the United States may be lost if the FDA
later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities
of the product to meet the needs of patients with the rare disease or condition.
Fast
Track Designation and Breakthrough Therapy Designation
The
FDA is required to facilitate the development, and expedite the review, of drugs that are intended for the treatment of a serious or
life-threatening disease or condition which demonstrate the potential to address unmet medical needs for the condition, and accordingly,
the FDA has established the fast track designation and breakthrough therapy designation programs.
A
product candidate is eligible for fast track designation if it is intended to treat a serious or life-threatening disease or condition
and demonstrates the potential to address unmet medical needs for such disease or condition. Fast track designation applies to the combination
of the product and the specific indication for which it is being studied. Under the fast track program, the sponsor of a drug candidate
may request that the FDA designate the candidate for a specific indication as a fast track product concurrent with, or after, the filing
of the IND for the candidate. The FDA must determine if the product candidate qualifies for fast track designation within 60 days of
receipt of the sponsor’s request. Fast track designation provides increased opportunities for sponsor interactions with the FDA
during preclinical and clinical development, in addition to the potential for rolling review of sections of the applicant’s NDA
or BLA before the application is complete. This rolling review is available if the applicant provides, and the FDA approves, a schedule
for the submission of the remaining information and the applicant pays applicable user fees. However, the FDA’s time period goal
for reviewing an application does not begin until the last section of the application is submitted. Additionally, the fast track designation
may be withdrawn by the FDA if the FDA believes that the designation is no longer supported by data emerging in the clinical trial process.
Under
the FDA’s breakthrough therapy program, a sponsor may seek FDA designation of its product candidate as a breakthrough therapy if
the product candidate is intended, alone or in combination with one or more other drugs or biologics, to treat a serious or life-threatening
disease or condition and preliminary clinical evidence indicates that it may demonstrate substantial improvement over existing therapies
on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. Breakthrough
therapy designation comes with all of the benefits of fast track designation. The FDA may take other actions appropriate to expedite
the development and review of the product candidate, including intensive guidance on an efficient product development program beginning
as early as Phase 1, and FDA organizational commitment to expedited development, including involvement of senior managers and experienced
review staff in a cross-disciplinary review, where appropriate.
Priority
Review
A
product is eligible for priority review if it has the potential to provide a significant improvement in safety or effectiveness in the
treatment, diagnosis or prevention of a serious disease or condition. A priority review means that the goal for the FDA to review an
application is six months, rather than the standard review of ten months under current PDUFA guidelines. Under the current PDUFA agreement,
these six-and ten-month review periods are measured from the “filing” date rather than the receipt date for NDAs for new
molecular entities, which typically adds approximately two months to the timeline for review and decision from the date of submission.
Most products that are eligible for fast track designation are also likely to be considered appropriate to receive a priority review.
Pediatric
Information
Under
the Pediatric Research Equity Act (the “PREA”), NDAs and BLAs, or supplements to NDAs and BLAs, must contain data to assess
the safety and effectiveness of the drug for the claimed indications in all relevant pediatric subpopulations and to support dosing and
administration for each pediatric subpopulation for which the drug is safe and effective. The FDA may grant full or partial waivers,
or deferrals, for submission of data. Unless otherwise required by regulation, PREA does not apply to any drug for an indication for
which orphan designation has been granted.
Disclosure
of Clinical Trial Information
Sponsors
of clinical trials of FDA-regulated products, including drugs and combination products, are required to register and disclose certain
clinical trial information. Information related to the product, patient population, phase of investigation, trial sites and investigators,
and other aspects of the clinical trial is then made public as part of the registration. Sponsors are also obligated to disclose the
results of their clinical trials after completion. Competitors may use this publicly available information to gain knowledge regarding
the progress of development programs. Disclosure of the results of these trials can be delayed until the new product or new indication
being studied has been approved. Failure to timely register a covered clinical study or to submit study results as provided for in the
law can give rise to civil monetary penalties and also prevent the non-compliant party from receiving future grant funds from the federal
government. The Final Rule on ClinicalTrials.gov registration and reporting requirements became effective in 2017, and both the National
Institutes of Health and the FDA have signaled the government’s willingness to begin enforcing those requirements against non-compliant
clinical trial sponsors.
Other
Regulatory Requirements
Health
Care Laws
Pharmaceutical
companies are subject to additional healthcare regulation and enforcement by the federal government and by authorities in the states
and foreign jurisdictions in which they conduct their business that may constrain the financial arrangements and relationships through
which Scienture researches,
as well as sell, market and distribute any products for which Scienture obtains marketing
authorization. Such laws include, without limitation, state and federal anti-kickback, fraud and abuse, false claims, and transparency
laws and regulations related to drug pricing and payments and other transfers of value made to physicians and other healthcare providers.
If Scienture’s operations are found to be in violation of any of such laws or any
other governmental regulations that apply, Scienture may be subject to penalties, including,
without limitation, administrative, civil and criminal penalties, damages, fines, disgorgement, the curtailment or restructuring of operations,
integrity oversight and reporting obligations, exclusion from participation in federal and state healthcare programs and responsible
individuals may be subject to imprisonment. Scienture may be subject to:
| |
● |
The
federal Anti-Kickback Statute, which prohibits, among other things, persons or entities from knowingly and willfully soliciting,
receiving, offering or paying any remuneration (including any kickback, bribe or rebate), directly or indirectly, overtly or covertly,
in cash or in kind, to induce, or in return for, the purchase, lease, order, arrangement, or recommendation of any good, facility,
item or service for which payment may be made, in whole or in part, under a federal healthcare program, such as the Medicare and
Medicaid programs. A person or entity does not need to have actual knowledge of the federal Anti-Kickback Statute or specific intent
to violate it to have committed a violation. Violations are subject to civil and criminal fines and penalties for each violation,
plus up to three times the remuneration involved, imprisonment, and exclusion from government healthcare programs. In addition, the
government may assert that a claim including items or services resulting from a violation of the federal Anti-Kickback Statute constitutes
a false or fraudulent claim for purposes of the federal False Claims Act or federal civil monetary penalties; |
| |
|
|
| |
● |
The
federal civil and criminal false claims laws and civil monetary penalty laws, such as the federal False Claims Act, which impose
criminal and civil penalties and authorize civil whistleblower or qui tam actions, against individuals or entities for, among other
things: knowingly presenting, or causing to be presented, to the federal government, claims for payment that are false or fraudulent;
knowingly making, using or causing to be made or used, a false statement of record material to a false or fraudulent claim or obligation
to pay or transmit money or property to the federal government or knowingly concealing or knowingly and improperly avoiding or decreasing
an obligation to pay money to the federal government. Manufacturers can be held liable under the federal False Claims Act even when
they do not submit claims directly to government payors if they are deemed to “cause” the submission of false or fraudulent
claims. The federal False Claims Act also permits a private individual acting as a “whistleblower” to bring actions on
behalf of the federal government alleging violations of the federal False Claims Act and to share in any monetary recovery; |
| |
|
|
| |
● |
The
federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), which created new federal criminal statutes
that prohibit a person from knowingly and willfully executing, or attempting to execute, a scheme to defraud any healthcare benefit
program or obtain, by means of false or fraudulent pretenses, representations or promises, any of the money or property owned by,
or under the custody or control of, any healthcare benefit program, regardless of the payor (e.g., public or private) and knowingly
and willfully falsifying, concealing or covering up by any trick or device a material fact or making any materially false, fictitious,
or fraudulent statements or representations in connection with the delivery of, or payment for, healthcare benefits, items or services
relating to healthcare matters; similar to the federal Anti-Kickback Statute, a person or entity does not need to have actual knowledge
of the statute or specific intent to violate it in order to have committed a violation; |
| |
● |
HIPAA,
as amended by the Health Information Technology for Economic and Clinical Health Act of 2009 (“HITECH”) and their respective
implementing regulations, including the Final Omnibus Rule published in January 2013, which impose requirements on certain covered
healthcare providers, health plans, and healthcare clearinghouses as well as their respective business associates, independent contractors
or agents of covered entities, that perform services for them that involve the creation, maintenance, receipt, use, or disclosure
of, individually identifiable health information relating to the privacy, security and transmission of individually identifiable
health information. HITECH also created new tiers of civil monetary penalties, amended HIPAA to make civil and criminal penalties
directly applicable to business associates, and gave state attorneys general new authority to file civil actions for damages or injunctions
in federal courts to enforce the federal HIPAA laws and seek attorneys’ fees and costs associated with pursuing federal civil
actions. In addition, there may be additional federal, state and non-U.S. laws which govern the privacy and security of health
and other personal information in certain circumstances, many of which differ from each other in significant ways and may not have
the same effect, thus complicating compliance efforts; |
| |
|
|
| |
● |
The
U.S. federal transparency requirements under the ACA, including the provision commonly referred to as the Physician Payments Sunshine
Act, and its implementing regulations, which requires applicable manufacturers of drugs, devices, biologics and medical supplies
for which payment is available under Medicare, Medicaid or the Children’s Health Insurance Program to report annually to the Centers for Medicare & Medicaid Services (“CMS”),
information related to payments or other transfers of value made to physicians (defined to include doctors, dentists, optometrists,
podiatrists and chiropractors), certain other licensed health care practitioners and teaching hospitals, as well as ownership and
investment interests held by the physicians described above and their immediate family members; |
| |
|
|
| |
● |
Federal
price reporting laws, which require manufacturers to calculate and report complex pricing metrics to government programs, where such
reported prices may be used in the calculation of reimbursement and/or discounts on approved products; and |
| |
|
|
| |
● |
Federal
consumer protection and unfair competition laws, which broadly regulate marketplace activities and activities that potentially harm
consumers. |
Additionally,
Scienture is subject to state and foreign equivalents of each of the healthcare laws and regulations described above, among others, some
of which may be broader in scope and may apply regardless of the payor. Many U.S. states have adopted laws similar to the federal Anti-Kickback
Statute and False Claims Act, and may apply to Scienture’s business practices, including, but not limited to, research, distribution,
sales or marketing arrangements and claims involving healthcare items or services reimbursed by non-governmental payors, including private
insurers. In addition, some states have passed laws that require pharmaceutical companies to comply with the April 2003 Office of Inspector
General Compliance Program Guidance for Pharmaceutical Manufacturers and/or the Pharmaceutical Research and Manufacturers of America’s
Code on Interactions with Healthcare Professionals. Several states also impose other marketing restrictions or require pharmaceutical
companies to make marketing or price disclosures to the state and require the registration of pharmaceutical sales representatives. There
are ambiguities as to what is required to comply with these state requirements and if Scienture fails to comply with an applicable state
law requirement Scienture could be subject to penalties. State and foreign laws, including for example the European Union General Data
Protection Regulation, which became effective in May 2018, also govern the privacy and security of health information in some circumstances,
many of which differ from each other in significant ways and often are not preempted by HIPAA, thus complicating compliance efforts.
There are ambiguities as to what is required to comply with these state requirements and if Scienture fails to comply with an applicable
state law requirement Scienture could be subject to penalties.
The
scope and enforcement of each of these laws is uncertain and subject to rapid change in the current environment of healthcare reform.
Federal and state enforcement bodies have recently increased their scrutiny of interactions between healthcare companies and healthcare
providers, which has led to a number of investigations, prosecutions, convictions and settlements in the healthcare industry.
Ensuring
that Scienture’s internal operations and future business arrangements with third parties comply with applicable healthcare laws
and regulations will involve substantial costs. It is possible that governmental authorities will conclude that Scienture’s business
practices do not comply with current or future statutes, regulations, agency guidance or case law involving applicable fraud and abuse
or other healthcare laws and regulations. If Scienture’s operations are found to be in violation of any of the laws described above
or any other governmental laws and regulations that may apply to us, Scienture may be subject to significant penalties, including administrative,
civil and criminal penalties, damages, fines, disgorgement, the exclusion from participation in federal and state healthcare programs,
individual imprisonment, reputational harm, and the curtailment or restructuring of Scienture’s operations, as well as additional
reporting obligations and oversight if Scienture becomes subject to a corporate integrity agreement or other agreement to resolve allegations
of non-compliance with these laws. Further, defending against any such actions can be costly and time consuming, and may require significant
financial and personnel resources. Therefore, even if Scienture is successful in defending against any such actions that may be brought
against it, its business may be impaired. If any of the physicians or other providers or entities with whom Scienture expects to do business
are found to not be in compliance with applicable laws, they may be subject to criminal, civil or administrative sanctions, including
exclusions from government funded healthcare programs and imprisonment. If any of the above occur, Scienture’s ability to operate
its business and its results of operations could be adversely affected.
Healthcare
Reform
Payors,
whether domestic or foreign, or governmental or private, are developing increasingly sophisticated methods of controlling healthcare
costs and those methods are not always specifically adapted for new technologies such as gene therapy and therapies addressing rare diseases
such as those Scienture is
developing. In both the United States and certain foreign jurisdictions, there have been a number of legislative and regulatory changes
to the health care system that could impact Scienture’s ability to sell its products
profitably. In particular, in 2010, the Patient Protection and Affordable Care Act, as amended by the Health Care
and Education Reconciliation Act of 2010 (collectively, the “ACA”) was enacted, which, among other things, subjected biologic products to potential competition
by lower-cost biosimilars; increased the minimum Medicaid rebates owed by most manufacturers under the Medicaid Drug Rebate Program;
extended the Medicaid Drug Rebate program to utilization of prescriptions of individuals enrolled in Medicaid managed care organizations;
subjected manufacturers to new annual fees and taxes for certain branded prescription drugs; created a Medicare Part D coverage gap discount
program, in which manufacturers must agree to offer 70% point-of-sale discounts off negotiated prices of applicable brand drugs
to eligible beneficiaries during their coverage gap period, as a condition for the manufacturer’s outpatient drugs to be covered
under Medicare Part D; and provided incentives to programs that increase the federal government’s comparative effectiveness research.
In
addition, other legislative and regulatory changes have been proposed and adopted in the United States since the ACA was enacted.
| |
● |
The
Budget Control Act of 2011 and subsequent legislation, among other things, created measures for spending reductions by Congress
that include aggregate reductions of Medicare payments to providers of 2% per fiscal year, which remain in effect through 2031. Due
to the Statutory Pay-As-You-Go Act of 2010, estimated budget deficit increases resulting from the American Rescue Plan Act of 2021,
and subsequent legislation, Medicare payments to providers will be further reduced starting in 2025 absent further legislation. The U.S.
American Taxpayer Relief Act of 2012 further reduced Medicare payments to several types of providers and increased the statute
of limitations period for the government to recover overpayments to providers from three to five years. |
| |
|
|
| |
● |
On
April 13, 2017, CMS published a final rule that gives states greater flexibility in setting benchmarks for insurers in the individual
and small group marketplaces, which may have the effect of relaxing the essential health benefits required under the ACA for plans
sold through such marketplaces. |
| |
|
|
| |
● |
On
May 30, 2018, the Right to Try Act, was signed into law. The law, among other things, provides a federal framework for certain patients
to access certain investigational new drug products that have completed a Phase 1 clinical trial and that are undergoing investigation
for FDA approval. Under certain circumstances, eligible patients can seek treatment without enrolling in clinical trials and without
obtaining FDA permission under the FDA expanded access program. There is no obligation for a pharmaceutical manufacturer to make
its drug products available to eligible patients as a result of the Right to Try Act. |
| |
|
|
| |
● |
On May
23, 2019, CMS published a final rule to allow Medicare Advantage Plans the option of using step therapy for Part B drugs. |
Additionally,
there has been increasing legislative and enforcement interest in the United States with respect to drug pricing practices. Specifically,
there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which
has resulted in several U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other
things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, and review the relationship between
pricing and manufacturer patient programs.
In
August 2022, the Inflation Reduction Act of 2022 (the “IRA”), was signed into law. The IRA includes several provisions that
may impact Scienture’s business, depending on how various aspects of the IRA are implemented. Provisions that may impact Scienture’s
business include a $2,000 out-of-pocket cap for Medicare Part D beneficiaries, the imposition of new manufacturer financial liability
on most drugs in Medicare Part D, permitting the U.S. government to negotiate Medicare Part B and Part D pricing for certain high-cost
drugs and biologics without generic or biosimilar competition, requiring companies to pay rebates to Medicare for drug prices that increase
faster than inflation, and delay until January 1, 2032 the implementation of the U.S. Department of Health and Human Services (“HHS”) rebate rule that would have limited the fees
that pharmacy benefit managers can charge. Further, under the IRA, orphan drugs are exempted from the Medicare drug price negotiation
program, but only if they have one orphan designation and for which the only approved indication is for that disease or condition.
If a product receives multiple orphan designations or has multiple approved indications, it may not qualify for the orphan drug exemption.
The implementation of the IRA is currently subject to ongoing litigation challenging the constitutionality of the IRA’s Medicare
drug price negotiation program. The effects of the IRA on Scienture’s
business and the healthcare industry in general is not yet known.
President
Biden has issued multiple executive orders that have sought to reduce prescription drug costs. In February 2023, HHS also issued
a proposal in response to an October 2022 executive order from President Biden that includes a proposed prescription drug pricing model
that will test whether targeted Medicare payment adjustments will sufficiently incentivize manufacturers to complete confirmatory trials
for drugs approved through FDA’s accelerated approval pathway. Although a number of these and other proposed measures may require
authorization through additional legislation to become effective, and the Biden administration may reverse or otherwise change these
measures, both the Biden administration and Congress have indicated that they will continue to seek new legislative measures to control
drug costs.
Scienture
expects that additional U.S. federal healthcare reform measures will be adopted in the future,
any of which could limit the amounts that the U.S. Federal Government will pay for healthcare drugs and services, which could result
in reduced demand for Scienture’s drug candidates or additional pricing pressures.
Individual
states in the United States have also become increasingly active in passing legislation and implementing regulations designed to control
pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain
drug access and marketing cost disclosure and transparency measures, and designed to encourage importation from other countries and bulk
purchasing. Legally mandated price controls on payment amounts by third-party payors or other restrictions could harm Scienture’s
business, financial condition, results of operations and prospects. In addition, regional healthcare authorities and individual hospitals
are increasingly using bidding procedures to determine what pharmaceutical products and which suppliers will be included in their prescription
drug and other healthcare programs. This could reduce the ultimate demand for Scienture’s drugs or put pressure on its drug pricing,
which could negatively affect its business, financial condition, results of operations and prospects.
Pharmaceutical
Coverage, Pricing, and Reimbursement
The
success of Scienture’s product candidates, if approved, depends on the availability of coverage and adequate reimbursement from
third-party payors. Scienture cannot be sure that coverage and reimbursement will be available for, or accurately estimate the potential
revenue from, its product candidates or assure that coverage and reimbursement will be available for any product that it may develop.
Patients who are provided medical treatment for their conditions generally rely on third-party payors to reimburse all or part of the
costs associated with their treatment. Coverage and adequate reimbursement from governmental healthcare programs, such as Medicare and
Medicaid, and commercial payors is critical to new product acceptance.
Government
authorities and other third-party payors, such as private health insurers and health maintenance organizations, decide which drugs and
treatments they will cover and the amount of reimbursement. Coverage and reimbursement by a third-party payor may depend upon a number
of factors, including the third-party payor’s determination that use of a product is:
| |
● |
a
covered benefit under its health plan; |
| |
|
|
| |
● |
safe,
effective and medically necessary; |
| |
|
|
| |
● |
appropriate
for the specific patient; |
| |
|
|
| |
● |
cost-effective;
and |
| |
|
|
| |
● |
neither
experimental nor investigational. |
In
the United States, no uniform policy of coverage and reimbursement for products exists among third-party payors. As a result, obtaining
coverage and reimbursement approval of a product from a government or other third-party payor is a time-consuming and costly process
that could require Scienture to provide to each payor supporting scientific, clinical and cost-effectiveness data for the use of Scienture’s
products on a payor-by-payor basis, with no assurance that coverage and adequate reimbursement will be obtained. In the United States,
the principal decisions about reimbursement for new medicines are typically made by CMS. CMS decides whether and to what extent a new
medicine will be covered and reimbursed under Medicare and private payors tend to follow CMS to a substantial degree. Even if Scienture
obtains coverage for a given product, the resulting reimbursement payment rates might not be adequate for Scienture to achieve or sustain
profitability or may require co-payments that patients find unacceptably high. Additionally, third-party payors may not cover, or provide
adequate reimbursement for, long-term follow-up evaluations required following the use of product candidates, once approved. Patients
are unlikely to use Scienture’s product candidates, once approved, unless coverage is provided and reimbursement is adequate to
cover a significant portion of their cost. There is significant uncertainty related to insurance coverage and reimbursement of newly
approved products. It is difficult to predict at this time what third-party payors will decide with respect to the coverage and reimbursement
for Scienture’s product candidates.
Net
prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by
any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in
the United States. Increasingly, third-party payors are requiring that drug companies provide them with predetermined discounts from
list prices and are challenging the prices charged for medical products. Scienture cannot be sure that reimbursement will be available
for any product candidate that it commercializes and, if reimbursement is available, the level of reimbursement. In addition, many pharmaceutical
manufacturers must calculate and report certain price reporting metrics to the government, such as average sales price and best price.
Penalties may apply in some cases when such metrics are not submitted accurately and timely. Further, these prices for drugs may be reduced
by mandatory discounts or rebates required by government healthcare programs. Payment methodologies may be subject to changes in healthcare
legislation and regulatory initiatives.
Scienture
expects that healthcare reform measures that may be adopted in the future may result in more rigorous coverage criteria and in additional
downward pressure on the price that Scienture receives for any approved product. The implementation of cost containment measures or other
healthcare reforms may prevent Scienture from being able to generate revenue, attain profitability, or commercialize Scienture’s
products. Legislative and regulatory proposals have been made to expand post-approval requirements and restrict sales and promotional
activities for pharmaceutical products. Scienture cannot be sure whether additional legislative changes will be enacted, or whether existing
regulations, guidance or interpretations will be changed, or what the impact of such changes on the marketing approvals or clearances
of Scienture’s product candidates, if any, may be.
In
addition, in some foreign countries, the proposed pricing for a drug must be approved before it may be lawfully marketed. The requirements
governing drug pricing vary widely from country to country. For example, the European Union provides options for its Member States to
restrict the range of medicinal products for which their national health insurance systems provide reimbursement and to control the prices
of medicinal products for human use. To obtain reimbursement or pricing approval, some of these countries may require the completion
of clinical trials that compare the cost effectiveness of a particular product candidate to currently available therapies. A Member State
may approve a specific price for the medicinal product or it may instead adopt a system of direct or indirect controls on the profitability
of the company placing the medicinal product on the market. There can be no assurance that any country that has price controls or reimbursement
limitations for pharmaceutical products will allow favorable reimbursement and pricing arrangements for any of Scienture’s product
candidates. Historically, products launched in the European Union do not follow price structures of the United States and generally prices
tend to be significantly lower.
Environmental
Matters
Scienture’s
operations and those of its third-party manufacturers and suppliers are subject to national, state and local environmental laws. Scienture
has made, and intends to continue to make, expenditures and undertake efforts to comply with applicable laws. Scienture believes the
safety procedures utilized by it for the handling and disposing hazardous materials comply with the standards prescribed by applicable
laws and regulations.
Human
Capital
Scienture’s
success begins and ends with our people. Scienture’s solid progress to date reflects the talent and hard work of all of its employees.
Scienture considers the intellectual capital of its employees to be an essential driver of its business and key to its future prospects.
Attracting, developing, and retaining talented people in technical, marketing, sales, research, and other positions is crucial to executing
its strategy and its ability to compete effectively.
Talent
Acquisition, Retention and Development
Scienture’s
key human capital objectives are to attract, retain and develop the highest quality talent. Scienture
employs various human resource programs in support of these objectives. Scienture’s
ability to recruit and retain such talent depends on a number of factors, including compensation
and benefits, talent development and career opportunities, and the work environment.
Scienture
attracts and rewards its employees by providing market competitive compensation and benefit packages,
including incentives and recognition plans that extend to all levels in its organization.
To that end, Scienture offers a comprehensive total rewards program aimed at health, home-life,
and financial needs of its employees. Scienture’s total rewards package includes market-competitive
pay, broad-based stock grants, bonuses, healthcare benefits, retirement savings plans, paid time off and family leave, an Employee Assistance
Program, and mental health services.
Scienture
is committed to the safety, health, and security of its employees. Scienture believes
a hazard-free environment is critical for the success of its business. Throughout Scienture’s operations,
Scienture strives to ensure that all its employees have access to safe workplaces that allow
them to succeed in their jobs. Scienture’s experience and continuing focus on workplace
safety has enabled it to preserve business continuity without sacrificing its commitment to keeping its colleagues and workplace visitors
safe.
Inclusion
and Diversity
Scienture
places a strong value on collaboration, inclusion, and diversity, and believes that working together
leads to better outcomes for its customers. This extends to the way Scienture employees treat
each other as team members. Scienture strives to create an environment where innovative
ideas can flourish by demonstrating respect for each other and valuing the diverse opinions, backgrounds, and viewpoints of employees.
Scienture believes a diverse and inclusive workplace results in business growth and encourages
increased innovation, retention of talent, and a more engaged workforce.
Facilities
Scienture
LLC’s corporate headquarters is located at 20 Austin Blvd, Commack, NY 11725, which is 2,000 square feet of office space with a
lease termination date of July 31, 2026. Scienture believes its facilities are sufficient to meet its current needs for the
foreseeable future.
Legal
Proceedings
From
time to time, Scienture may be involved in various claims and legal proceedings. Scienture is not currently a party to any material legal
proceedings.
Employees
Currently,
the Company and Scienture LLC collectively employ approximately fourteen (14) full-time employees and five (5) part-time employees.
We are not a party to any collective bargaining agreements and have not experienced any strikes or work stoppages. We consider our
relations with our employees and consultants to be satisfactory.
Seasonality
Our
business is not directly affected by seasonal fluctuations but is affected indirectly by the fall and winter flu season, to the extent
it leads to an increased demand for certain generic pharmaceuticals.
PLAN
OF DISTRIBUTION
The
Selling Stockholders, which as used in this prospectus includes donees, pledgees, transferees or other successors-in-interest
selling shares or interests in shares received after the date of this prospectus from the Selling Stockholders as a gift, pledge,
partnership distribution or other transfer, may, from time to time, sell, transfer or otherwise dispose of any or all of the shares covered
hereby on any stock exchange, market or trading facility on which the shares are traded or in private transactions. These dispositions
may be at fixed prices, at prevailing market prices at the time of sale, at prices related to the prevailing market price, at varying
prices determined at the time of sale, or at negotiated prices. The Selling Stockholders may use any one or more of the following
methods when selling the shares, unless they are contractually bound not to:
| |
● |
ordinary
brokerage transactions and transactions in which the broker-dealer solicits purchasers; |
| |
|
|
| |
● |
block
trades in which the broker-dealer will attempt to sell the securities as agent but may position and resell a portion of the block
as principal to facilitate the transaction; |
| |
|
|
| |
● |
purchases
by a broker-dealer as principal and resale by the broker-dealer for its account; |
| |
|
|
| |
● |
an
exchange distribution in accordance with the rules of the applicable exchange; |
| |
|
|
| |
● |
privately
negotiated transactions; |
| |
|
|
| |
● |
settlement
of short sales; |
| |
|
|
| |
● |
in
transactions through broker-dealers that agree with the Selling Stockholders to sell a specified number of shares at a stipulated
price per security; |
| |
|
|
| |
● |
through
the writing or settlement of options or other hedging transactions, whether through an options exchange or otherwise; |
| |
|
|
| |
● |
a
combination of any such methods of sale; or |
| |
|
|
| |
● |
any
other method permitted pursuant to applicable law. |
The
Selling Stockholders may also sell the shares under Rule 144 or any other exemption from registration under the Securities Act,
if available, rather than under this prospectus.
Broker-dealers
engaged by the Selling Stockholders may arrange for other brokers-dealers to participate in sales. Broker-dealers may receive
commissions or discounts from the Selling Stockholders (or, if any broker-dealer acts as agent for the purchaser of the shares,
from the purchaser) in amounts to be negotiated, but, except as set forth in a supplement to this prospectus, in the case of an agency
transaction not in excess of a customary brokerage commission in compliance with FINRA Rule 2121; and in the case of a principal transaction
a markup or markdown in compliance with FINRA Rule 2121.
In
connection with the sale of the shares or interests therein, the Selling Stockholders may enter into hedging transactions with
broker-dealers or other financial institutions, which may in turn engage in short sales of the shares in the course of hedging the positions
they assume. The Selling Stockholders may also sell shares short and deliver these shares to close out their short positions,
or loan or pledge the shares to broker-dealers that in turn may sell these shares. The Selling Stockholders may also enter into
option or other transactions with broker-dealers or other financial institutions or create one or more derivative securities which require
the delivery to such broker-dealer or other financial institution of shares offered by this prospectus, which shares such broker-dealer
or other financial institution may resell pursuant to this prospectus (as supplemented or amended to reflect such transaction).
Each
Selling Stockholder is, and any broker-dealer or
agent that is involved in selling the shares may be deemed to be, an “underwriter” within the meaning of the Securities
Act in connection with such sales. In such event, any commissions received by such broker-dealers or agents and any profit on the resale
of the shares purchased by them may be deemed to be underwriting commissions or discounts under the Securities Act. Each Selling
Stockholder has informed the Company that it does not have any written or oral agreement or understanding, directly or indirectly, with
any person to distribute the shares.
The
Company is required to pay certain fees and expenses incurred by the Company incident to the registration of the shares. The Company
has agreed to indemnify the Selling Stockholders against certain losses, claims, damages and liabilities, including liabilities
under the Securities Act.
We
agreed to keep this prospectus effective until all of the shares have been sold pursuant to this prospectus or Rule 144 under the Securities
Act or any other rule of similar effect. The shares will be sold only through registered or licensed brokers or dealers if required under
applicable state securities laws. In addition, in certain states, the shares covered hereby may not be sold unless they have been registered
or qualified for sale in the applicable state or an exemption from the registration or qualification requirement is available and is
complied with.
Compliance
with the Exchange Act, including Regulation M
Under
applicable rules and regulations under the Exchange Act, any person engaged in the distribution of the shares may not simultaneously
engage in market making activities with respect to the common stock for the applicable restricted period, as defined in Regulation M,
prior to the commencement of the distribution. In addition, the Selling Stockholder will be subject to applicable provisions of the Exchange
Act and the rules and regulations thereunder, including Regulation M, which may limit the timing of purchases and sales of the common
stock by the Selling Stockholders or any other person.
DESCRIPTION
OF cAPITAL sTOCK
The
following summary describes the common stock of the Company, which common stock is registered pursuant to Section 12 of the Exchange
Act. Only the Company’s common stock is registered under Section 12 of the Exchange Act.
The
following description of our common stock is a summary and is qualified in its entirety by reference to our certificate of incorporation,
as amended and our bylaws, as amended, which are incorporated by reference herein, and by applicable law. For purposes of this description,
references to the “Company,” “we,” “our” and “us” refer only to the Company and not to
its subsidiaries.
Authorized
Capitalization
The
total number of authorized shares of our common stock is 100,000,000 shares, $0.00001 par value per share. The total number of authorized
shares of our preferred stock is 10,000,000 shares, $0.00001 par value per share.
Common
Stock
Voting
Rights. Each share of our common stock is entitled to one vote on all stockholder matters. Shares of our common stock do not
possess any cumulative voting rights. Except for the election of directors, if a quorum is present, an action on a matter is approved
if it receives the affirmative vote of the holders of a majority of the voting power of the shares of capital stock present in person
or represented by proxy at the meeting and entitled to vote on the matter, unless otherwise required by applicable law, Delaware law,
our certificate of incorporation, as amended or bylaws, as amended. The election of directors will be determined by a plurality of the
votes cast in respect of the shares present in person or represented by proxy at the meeting and entitled to vote, meaning that the nominees
with the greatest number of votes cast, even if less than a majority, will be elected. The rights, preferences and privileges of holders
of common stock are subject to, and may be impacted by, the rights of the holders of shares of any series of preferred stock that we
have designated, or may designate and issue in the future.
Dividend
Rights. Each share of our common stock is entitled to equal dividends and distributions per share with respect to the common
stock when, as and if declared by our board of directors, subject to any preferential or other rights of any outstanding preferred stock.
Liquidation
and Dissolution Rights. Upon liquidation, dissolution or winding up, our common stock will be entitled to receive pro rata on
a share-for-share basis, the assets available for distribution to the stockholders after payment of liabilities and payment of preferential
and other amounts, if any, payable on any outstanding preferred stock.
Fully
Paid Status. All outstanding shares of the Company’s common stock are validly issued, fully paid and non-assessable.
Listing. Our common stock is listed and traded on Nasdaq under the symbol “SCNX”.
Other
Matters. No holder of any shares of our common stock has a preemptive right to subscribe for any of our securities, nor are any
shares of our common stock subject to redemption or convertible into other securities.
Anti-Takeover
Effects Under Section 203 of Delaware General Corporation Law, our Certificate of Incorporation and Bylaws
Section
203 of Delaware General Corporation Law (DGCL) prohibits a Delaware corporation from engaging in any business combination with any interested
stockholder for a period of three years after the date that such stockholder became an interested stockholder, with the following exceptions:
| |
● |
before
such date, the board of directors of the corporation approved either the business combination or the transaction that resulted in
the stockholder becoming an interested stockholder; |
| |
|
|
| |
● |
upon
completion of the transaction that resulted in the stockholder becoming an interested stockholder, the interested stockholder owned
at least eighty five percent (85%) of the voting stock of the corporation outstanding at the time the transaction began, excluding
for purposes of determining the voting stock outstanding (but not the outstanding voting stock owned by the interested stockholder)
those shares owned (i) by persons who are directors and also officers and (ii) employee stock plans in which employee participants
do not have the right to determine confidentially whether shares held subject to the plan will be tendered in a tender or an exchange
offer; or |
| |
|
|
| |
● |
on
or after such date, the business combination is approved by our board of directors and authorized at an annual or a special meeting
of the stockholders, and not by written consent, by the affirmative vote of at least 66 2/3 percent of the outstanding voting stock
that is not owned by the interested stockholder. |
In
general, Section 203 defines “business combination” to include the following:
| |
● |
any
merger or consolidation involving the corporation or any direct or indirect majority owned subsidiary of the corporation and the
interested stockholder or any other corporation, partnership, unincorporated association, or other entity if the merger or consolidation
is caused by the interested stockholder and as a result of such merger or consolidation the transaction is not excepted as described
above; |
| |
● |
any
sale, transfer, pledge, or other disposition (in one transaction or a series) of ten percent (10%) or more of the assets of the corporation
involving the interested stockholder; |
| |
|
|
| |
● |
subject
to certain exceptions, any transaction that results in the issuance or transfer by the corporation of any stock of the corporation
to the interested stockholder; |
| |
|
|
| |
● |
any
transaction involving the corporation that has the effect of increasing the proportionate share of the stock or any class or series
of the corporation beneficially owned by the interested stockholder; or |
| |
|
|
| |
● |
the
receipt by the interested stockholder of the benefit of any loss, advances, guarantees, pledges, or other financial benefits by or
through the corporation. |
In
general, Section 203 defines an “interested stockholder” as an entity or a person who, together with the person’s affiliates
and associates, beneficially owns, or within three years prior to the time of determination of interested stockholder status did own,
fifteen percent (15%) or more of the outstanding voting stock of the corporation.
A
Delaware corporation may “opt out” of these provisions with an express provision in its Certificate of Incorporation. Our
Certificate of Incorporation provides that we shall not be governed by Section 203 of DGCL and as a result, Section 203 of DGCL does
not apply to us.
Our
certificate of incorporation does not provide that our board of directors will be classified. As a result, a person can gain control
of our board only by successfully engaging in a proxy contest at one annual meeting.
Our
authorized but unissued common stock and preferred stock are available for future issuances without stockholder approval and could be
utilized for a variety of corporate purposes, including future offerings to raise additional capital, acquisitions and employee benefit
plans. The existence of authorized but unissued and unreserved common stock and preferred stock could render more difficult or discourage
an attempt to obtain control of us by means of a proxy contest, tender offer, merger or otherwise.
Exclusive
forum for certain lawsuits
Our
bylaws require, that unless the Company consents in writing to an alternative forum, the Court of Chancery of the State of Delaware
shall, to the fullest extent permitted by law, be the sole and exclusive forum for (a) any derivative action or proceeding brought on
behalf of the Company; (b) any action asserting a claim of breach of fiduciary duty owed by, or other wrongdoing by, any director, officer,
employee or agent of the Company to the Company or the Company’s stockholders; (c) any action asserting a claim arising pursuant
to any provision of the DGCL or the certificate of incorporation or bylaws of the Company; (d) any action to interpret, apply, enforce
or determine the validity of the certificate of incorporation or bylaws of the Company; or (e) any action asserting a claim governed
by the internal affairs doctrine, in each case subject to said Court of Chancery having personal jurisdiction over the indispensable
parties named as defendants therein (or such indispensable parties consenting to the personal jurisdiction of the Court of Chancery within
10 days following any determination by the Court of Chancery that an indispensable party is not subject to such personal jurisdiction);
provided that, if and only if the Court of Chancery of the State of Delaware dismisses any action for lack of subject matter jurisdiction,
such action may be brought in another state or federal court sitting in the State of Delaware.
Notwithstanding
any other provisions of law, the certificate of incorporation or the bylaws of the Company, and notwithstanding the fact that a lesser
percentage may be specified by law, the affirmative vote of the holders of at least two-thirds in voting power of the outstanding shares
of capital stock of the Company entitled to vote thereon shall be required to amend or repeal, or to adopt any provision inconsistent
with the exclusive forum requirements in our certificate of incorporation. If any provision or provision of the exclusive forum requirements
in our certificate of incorporation shall be held to be invalid, illegal or unenforceable as applied to any person or entity or circumstance
for any reason whatsoever, then, to the fullest extent permitted by law, the validity, legality and enforceability of such provisions
in any other circumstance and of the remaining provisions and the application of such provision to other persons or entities and circumstances
shall not in any way be affected or impaired thereby.
As
a result of the above, our certificate of incorporation provides that the exclusive forum provision will be applicable to the fullest
extent permitted by applicable law, subject to certain exceptions. However, Section 27 of the Exchange Act creates exclusive federal
jurisdiction over all suits brought to enforce any duty or liability created by the Exchange Act or the rules and regulations thereunder.
As a result, the exclusive forum provision will not apply to suits brought to enforce any duty or liability created by the Exchange Act
or any other claim for which the federal courts have exclusive jurisdiction. We also note that investors cannot waive compliance with
the federal securities laws and the rules and regulations thereunder. Section 22 of the Securities Act, creates concurrent jurisdiction
for state and federal courts over all suits brought to enforce any duty or liability created by the Securities Act or the rules and regulations
thereunder. The Company believes that the exclusive forum provision applies to claims arising under the Securities Act, but there
is uncertainty as to whether a court would enforce such a provision in this context.
Special
meeting of stockholders
Our
bylaws provide that special meetings of our stockholders may be called only by the chairperson of the board of directors, the chief executive
officer or president (in the absence of a chief executive officer). Because our stockholders do not have the right to call a special
meeting, a stockholder could not force stockholder consideration of a proposal over the opposition of our board of directors by calling
a special meeting of stockholders prior to such time as the chairperson of the board of directors, the chief executive officer or president
(in the absence of a chief executive officer) believed the matter should be considered or until the next annual meeting provided that
the requestor met the notice requirements. The restriction on the ability of stockholders to call a special meeting means that a proposal
to replace our board of directors also could be delayed until the next annual meeting.
Advance
notice requirements for stockholder proposals and director nominations
Our
bylaws provide that stockholders seeking to bring business before our annual meeting of stockholders, or to nominate candidates for election
as directors at our annual meeting of stockholders, must provide timely notice of their intent in writing. Separately, pursuant to Rule
14a-8 of the Exchange Act, proposals seeking inclusion in our annual proxy statement must comply with the notice periods contained therein.
Our Bylaws also specify certain requirements as to the form and content of a stockholders’ meeting. These provisions may preclude
our stockholders from bringing matters before our annual meeting of stockholders or from making nominations for directors at our annual
meeting of stockholders and may also discourage or deter a potential acquirer from conducting a solicitation of proxies to elect the
acquirer’s own slate of directors or otherwise attempting to obtain control of our company.
Action
by written consent
Any
action required or permitted to be taken by our common stockholders may be effected by written consent of the stockholders having not
less than the minimum percentage of the vote required by DGCL for the proposed corporate action.
Vacancies
on the Board of Directors
Our
bylaws provide that, subject to the rights of the holders of any outstanding series of preferred stock and unless otherwise required
by law or resolution of our board of directors, vacancies on the board of directors arising through death, resignation, retirement, disqualification
or removal, an increase in the number of directors or otherwise may be filled by a majority of the directors then in office, though less
than a quorum.
Amendment
to Bylaws by Stockholders
Subject
to certain limitations preventing amendments which decrease or diminish indemnification rights provided for in our bylaws, our bylaws
provide that any amendment to such bylaws undertaken solely by our stockholders requires the affirmative vote of at least two-thirds
in voting power of the outstanding shares of capital stock of the Company.
EXECUTIVE
COMPENSATION
2024
Summary Compensation Table
The
following table sets forth certain information concerning compensation earned by or paid to certain persons who we refer to as our “Named
Executive Officers” for services provided for the fiscal years ended December 31, 2024 and 2023.
| Name
and Principal Position | |
Year | |
Salary ($) | | |
Bonus ($) | | |
Stock
Awards ($)* | | |
Option
Awards ($)* | | |
All
Other Compensation ($) | | |
Total ($) | |
| Suren Ajjarapu | |
2024 | |
$ | 484,154 | | |
| - | | |
| 25,500 | | |
| - | | |
| 60,923 | (1) | |
$ | 570,577 | |
| Chairman
of the Board, Chief Executive Officer, and Secretary | |
2023 | |
$ | 360,000 | | |
| - | | |
| 243,075 | | |
$ | - | | |
| 24,934 | (1) | |
$ | 628,009 | |
| | |
| |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Prashant Patel | |
2024 | |
$ | 332,962 | | |
| - | | |
| 76,500 | | |
| - | | |
| - | | |
$ | 409,462 | |
| President,
Chief Operating Officer, Interim Principal Financial/ Accounting Officer and Director | |
2023 | |
$ | 150,000 | | |
| - | | |
| 43,650 | | |
$ | - | | |
| - | | |
$ | 193,650 | |
| * |
Amounts
in this column represent the aggregate grant date fair value of awards computed in accordance with Financial Accounting Standards
Board Accounting Standard Codification Topic 718. Such grant date fair value does not take into account any estimated forfeitures.
The assumptions used in calculating the grant date fair value of restricted shares and option awards are set forth in the Critical
Accounting Estimates as disclosed in our Consolidated Financial Statements for the year ended December 31, 2024. The amount reported
in this column reflects the accounting cost for these awards and does not correspond to the actual economic value that may be received
by the officer upon the vesting of the restricted shares, the exercise of the stock options, or any sale of the underlying shares
of common stock. |
| |
|
| (1) |
Represents
a car allowance of $1,000 per month and a disability insurance policy paid for by the Company. |
Narrative
Disclosure to 2024 Summary Compensation Table
Elements
of Compensation
The
compensation of our named executive officers generally consists of base salary and long-term incentive compensation in the form of equity
awards and other benefits, as described below.
2023
Increased Officer Compensation
Effective
January 1, 2023, the Board and the Compensation Committee, increased the annual salaries of each of Mr. Ajjarapu, Mr. Patel and Ms. Huffman
to the levels of their salaries prior to certain reductions that had been effective since September 1, 2022. Mr. Ajjarapu’s annual
salary was increased back to $360,000 per year, Mr. Patel’s annual salary was increased back to $150,000 per year.
The
increases in officer salaries were documented by amendments to the employment agreements with each officer. The amendments also clarified
that the equity compensation issuable to each officer was additional compensation and not specifically a result of the reduction in salaries
effective on September 1, 2022, and that the amount of reduced salary from September 1, 2022, to December 31, 2022 was forgiven by each
officer.
Outstanding
Equity Awards At Fiscal Year-End
The
following table sets forth information as of December 31, 2024, concerning unexercised options, unvested stock and equity incentive plan
awards for each of the executive officers named in the Summary Compensation Table.
| Name |
|
Grant
Date |
|
|
Number
of Securities Underlying Unexercised Options (#) Exercisable |
|
|
Number
of Securities Underlying Unexercised Options (#) Unexercisable |
|
|
Equity
Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options |
|
|
Option
Exercise Price ($) |
|
|
Option
Expiration Date |
|
| Suren Ajjarapu |
|
|
5/13/2019 |
|
|
|
1,111 |
|
|
|
- |
|
|
|
- |
|
|
$ |
39.60 |
|
|
|
5/13/2029 |
|
| |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Prashant Patel |
|
|
5/13/2019 |
|
|
|
1,111 |
|
|
|
- |
|
|
|
- |
|
|
$ |
39.60 |
|
|
|
5/13/2029 |
|
Employment
Agreements with Our Named Executive Officers
Suren
Ajjarapu, Chief Executive Officer and Secretary
Effective
on April 14, 2020, we entered into an employment agreement with Mr. Suren Ajjarapu, our Chief Executive Officer, which replaced and superseded
his prior employment agreement with the Company.
The
agreement, which provides for Mr. Ajjarapu to serve as our Chief Executive Officer, has a term extending through December 31, 2025, provided
that the agreement automatically extends for additional one-year terms thereafter in the event neither party provides the other at least
60 days prior notice of their intention not to renew the terms of the agreement. The agreement also requires the Board, subject to certain
exceptions, to nominate Mr. Ajjarapu to serve on the Board at each stockholders’ meeting which occurs during the term of the agreement
and to serve as the Chairman of the Board.
Pursuant
to the terms of the agreement, Mr. Ajjarapu’s annual compensation package includes (1) a base salary of $360,000 per year ($300,000
for the 2020 fiscal year), subject to annual increases as determined in the sole discretion of the Compensation Committee of the Board
(the “Compensation Committee”), and as discussed below (the “Base Salary”), and (2) a performance bonus equal
to up to 100% of his Base Salary each year, based on the Company meeting certain performance metrics as determined from time to time
by the Compensation Committee and Mr. Ajjarapu (“Performance Metrics”). Additionally, in the event that Mr. Ajjarapu meets
at least 70% of the requirements for any annual performance bonus, as determined in the reasonable discretion of the Compensation Committee
of the Board (which requirement was met for the 2020 fiscal year, and which salary was automatically increased), Mr. Ajjarapu’s
Base Salary is increased by 20%. Mr. Ajjarapu is eligible for the Base Salary increase on an annual basis, with such increases being
cumulative. Such increases in Base Salary do not require an amendment to the agreement. Mr. Ajjarapu’s performance bonus metrics
include specific company performance goals and objectives, including revenue goals, app downloads, and net operating income milestones,
as may be modified or added to from time to time with the mutual approval of Mr. Ajjarapu and the Compensation Committee. The determination
of whether the Performance Metrics have been met are determined in the reasonable discretion of the Compensation Committee, no later
than 90 days after (a) December 31, 2020, in connection with the 2020 Performance Metrics; and (b) the end of such calendar year for
subsequent years. For the year ended December 31, 2020, Mr. Ajjarapu was awarded 49,020 shares of restricted common stock (the “2020
Restricted Stock”), valued at $372,062, based on the closing sales price of the Company’s common stock on the effective date
of grant, which vested in full. Mr. Ajjarapu may also receive additional bonuses awarded from time to time in the discretion of the Board
and/or Compensation Committee and the Board (in cash, options or other forms of equity) or the Compensation Committee may waive or change
the performance metrics associated with his performance bonus in their discretion. Mr. Ajjarapu’s compensation under his employment
agreement may be increased from time to time, by the Compensation Committee, or the Board (with the recommendation of the Compensation
Committee), which increases do not require the entry into an amended employment agreement. Mr. Ajjarapu is also paid an automobile allowance
of $1,000 per month during the term of the agreement and is eligible to participate in our stock option plan and other benefit plans.
The
agreement requires Mr. Ajjarapu to devote at least 75% of his business time and efforts to Company business. The agreement also prohibits
Mr. Ajjarapu from competing against us during the term of the agreement and for a period of twelve months after the termination of the
agreement in any state and any other geographic area in which we or our subsidiaries provide Restricted Services or Restricted Products,
directly or indirectly, during the twelve months preceding the date of the termination of the agreement. “Restricted Services”
means the manufacture, distribution, wholesale and sale of Restricted Products, healthcare services and any other services that we or
our subsidiaries have provided or are researching, developing, performing and/or providing at any time during the two years immediately
preceding the date of termination, or which Mr. Ajjarapu has obtained any trade secret or other confidential information about at any
time during the two years immediately preceding the date of termination of the agreement. “Restricted Products” means pharmaceutical
drugs and other healthcare products and any other product, that we or our subsidiaries have provided or are researching, developing,
manufacturing, distributing, purchasing, selling and/or providing at any time during the two years immediately preceding the date the
agreement is terminated, or which Mr. Ajjarapu obtained any trade secret or other confidential information in connection with at any
time during the two years immediately preceding the date of termination of the agreement.
We
may terminate Mr. Ajjarapu’s employment (a) for “cause” (which is defined to include, a material breach of the agreement
by Mr. Ajjarapu, any act of misappropriation of funds or embezzlement by Mr. Ajjarapu, Mr. Ajjarapu committing any act of fraud, or Mr.
Ajjarapu being indicted of, or pleading guilty or nolo contendere with respect to, theft, fraud, a crime involving moral turpitude, or
a felony under federal or applicable state law); (b) in the event Mr. Ajjarapu suffers a physical or mental disability which renders
him unable to perform his duties and obligations for either 90 consecutive days or 180 days in any 12-month period; (c) for any reason
without “cause”; or (d) upon expiration of the initial term of the agreement (or any renewal) upon notice as provided above.
The agreement also automatically terminates upon the death of Mr. Ajjarapu.
Mr.
Ajjarapu may terminate his employment (a) for “good reason” (i.e., (i) if his position or duties are modified to such an
extent that his duties are no longer consistent with the position of CEO of the Company, (ii) there has been a material breach by us
of a material term of the agreement or Mr. Ajjarapu reasonably believes that we are violating any law which would have a material adverse
effect on our operations and such violation continues uncured thirty days after such breach and after notice thereof has been provided
to us by Mr. Ajjarapu, (iii) Mr. Ajjarapu’s compensation is reduced without his consent, or we fail to pay to Mr. Ajjarapu any
compensation due to him upon five days written notice from Mr. Ajjarapu informing us of such failure, or (iv) if Mr. Ajjarapu is also
then serving as a member of the Board and is not re-nominated by the Board to serve as a member of the Board at any annual meeting of
stockholders of the Company; provided, however, prior to any such termination by Mr. Ajjarapu for “good reason”, Mr. Ajjarapu
must first advise us in writing (within 15 days of the occurrence of such event) and provide us 15 days to cure (5 days in connection
with the reduction of Mr. Ajjarapu’s salary or the failure to pay amounts owed to him)); (b) for any reason without “good
reason”; and (c) upon expiration of the initial term of the agreement (or any renewal) upon notice as provided above.
In
the event that Mr. Ajjarapu’s employment is terminated for any reason (not including, however, a termination by us for “cause”
or a termination as a result of Mr. Ajjarapu’s death or disability) during the twelve month period following a Change of Control
(a “Change of Control Termination”) or in anticipation of a Change of Control, we are required to pay Mr. Ajjarapu, within
60 days following the later of (i) the date of such Change of Control Termination; and (ii) the date of such Change of Control, a cash
severance payment in a lump sum in an amount equal to 3.0 times the sum of his current base salary and the amount of the last bonus payable
to Mr. Ajjarapu (the “Change of Control Payment”), which amount is due within 60 days of the later of (i) the date of such
Change of Control Termination; and (ii) the date of such Change of Control. If Mr. Ajjarapu’s employment terminates due to a Change
of Control Termination within six (6) months prior to a Change of Control, it will be deemed to be “in anticipation of a Change
of Control” for all purposes. In addition, in the event of a Change of Control, all of Mr. Ajjarapu’s equity-based compensation
immediately vests to Mr. Ajjarapu and any outstanding stock options held by Mr. Ajjarapu can be exercised by Mr. Ajjarapu until the earlier
of (A) one (1) year from the date of termination and (B) the latest date upon which such stock options would have expired by their original
terms under any circumstances, provided that if Mr. Ajjarapu’s employment ends in anticipation of a Change of Control and such
equity-based compensation awards or stock options have previously expired pursuant to their terms, the Company is required to pay Mr.
Ajjarapu a lump sum payment, payable on the same date as the Change of Control Payment, equal to the Black Scholes value of the expired
and unexercised equity compensation awards and stock options held by Mr. Ajjarapu on the date of termination, based on the value of such
awards had they been exercisable through the end of their stated term and had not previously expired. “Change of Control”
for the purposes of the agreement means: (a) any person obtaining beneficial ownership representing more than 50% of the total voting
power represented by our then outstanding voting securities without the approval of not fewer than two-thirds of our Board; (b) a merger
or consolidation of us whether or not approved by our Board, other than a merger or consolidation that would result in our voting securities
immediately prior thereto continuing to represent at least 50% of the total voting power outstanding immediately after such merger or
consolidation, (c) our stockholders approving a plan of complete liquidation or an agreement for the sale or disposition by us of all
or substantially all of our assets, or (d) as a result of the election of members to our Board, a majority of the Board consists of persons
who are not members of the Board on April 14, 2020, except in the event that such slate of directors is proposed by a committee of the
Board or the Board; provided that if the definition of “Change of Control” in our Stock Incentive Plans or Equity Compensation
Plans is more favorable than the definition above, then such definition shall be controlling.
If
Mr. Ajjarapu’s employment is terminated pursuant to his death, disability, the end of the initial term (or any renewal term), without
“good reason” by Mr. Ajjarapu, or by us for “cause”, Mr. Ajjarapu is entitled to all salary accrued through the
termination date and no other benefits other than as required under the terms of employee benefit plans in which Mr. Ajjarapu was participating
as of the termination date. Additionally, any unvested stock options or equity compensation held by Mr. Ajjarapu immediately terminate
and are forfeited (unless otherwise provided in the applicable award) and any previously vested stock options (or if applicable equity
compensation) are subject to the terms and conditions set forth in the applicable Stock Incentive Plan or Equity Compensation Plan, or
award agreement, as such may describe the rights and obligations upon termination of employment of Mr. Ajjarapu.
If
Mr. Ajjarapu’s employment is terminated by Mr. Ajjarapu for “good reason”, or by us without “cause”, Mr.
Ajjarapu is entitled to continue to receive the salary due pursuant to the terms of the agreement at the rate in effect upon the termination
date for eighteen (18) months, plus the pro rata amount of any discretionary bonus and performance bonus he would have been due for the
following eighteen (18) months (with any metrics being extrapolated based on the last four (4) full prior quarters of the Company’s
operations prior to termination). Additionally, unvested benefits (whether equity or cash benefits and bonuses) will vest immediately
upon such termination and any outstanding stock options previously granted to Mr. Ajjarapu will vest immediately upon such termination
and will be exercisable until the earlier of (A) one year from the date of termination and (B) the latest date upon which such stock
options would have expired by their original terms under any circumstances. Mr. Ajjarapu is also to receive, if he elects, continued
health insurance under COBRA, paid for by the Company, for eighteen (18) months following the termination date (subject to certain rights
which reduce such obligation if Mr. Ajjarapu is covered by health insurance with a substantially similar level of insurance as prior
to the termination).
The
agreement contains standard assignment of inventions, indemnification and confidentiality provisions. Further, Mr. Ajjarapu is subject
to non-solicitation covenants during the term of the agreement.
Although
Mr. Ajjarapu will be prohibited from competing with us while he is employed with us, he will only be prohibited from competing for twelve
months after his employment with us ends pursuant to the agreement.
See
also “2022 Reduced Officer Compensation” and “2023 Increased Officer Compensation” above.
Prashant
Patel, President, Chief Operating Officer and Interim Principal Financial/Accounting Officer
Effective
March 31, 2024, we entered into an employment agreement with Mr. Prashant Patel, our President, Chief Operating Officer and Interim Principal
Financial/Accounting Officer, which replaced and superseded his prior employment agreement with the Company.
The
agreement, which provides for Mr. Patel to serve as our President Chief Operating Officer, has a term extending through December 31,
2025, provided that the agreement automatically extends for additional one-year terms thereafter in the event neither party provides
the other at least 60 days prior notice of their intention not to renew the terms of the agreement. The agreement also requires the Board,
subject to certain exceptions, to nominate Mr. Patel to serve on the Board at each stockholders’ meeting which occurs during the
term of the agreement.
Pursuant
to the terms of the agreement, Mr. Patel’s annual compensation package includes (1) a base salary of $350,000 per year, subject
to annual increases as determined in the sole discretion of the Chief Executive Officer, and as discussed below (the “Base Salary”),
and (2) a performance bonus equal to up to 100% of his Base Salary each year, based on the Company meeting certain performance metrics
as determined from time to time by the Compensation Committee of the Board (“Performance Metrics”). Additionally, in the
event that Mr. Patel meets at least 70% of the requirements for any annual performance bonus, as determined in the reasonable discretion
of the Compensation Committee of the Board (the “Compensation Committee”), Mr. Patel’s Base Salary will increase by
20%. Mr. Patel is eligible for the Base Salary increase on an annual basis, with such increases being cumulative. Such increases in Base
Salary do not require an amendment to the agreement. Mr. Patel’s performance bonus metrics are to be added to the agreement and
the Company currently contemplates that such metrics will include specific company performance goals and objectives, including revenue
goals, app downloads, and net operating income milestones, as may be modified or added to from time to time with the mutual approval
of Mr. Patel and the Compensation Committee. The determination of whether the Performance Metrics have been met are determined in the
reasonable discretion of the Compensation Committee, no later than 90 days after the end of such calendar year. Mr. Patel may also receive
additional bonuses awarded from time to time in the discretion of the Compensation Committee (in cash, options or other forms of equity).
Mr. Patel is also paid an automobile allowance of $1,000 per month during the term of the agreement and is eligible to participate in
our stock option plan and other benefit plans.
The
agreement requires Mr. Patel to devote at least 75% of his business time and efforts to Company business. The agreement also prohibits
Mr. Patel from competing against us during the term of the agreement and for a period of twelve months after the termination of the agreement
in any state and any other geographic area in which we or our subsidiaries provide Restricted Services or Restricted Products, directly
or indirectly, during the twelve months preceding the date of the termination of the agreement. “Restricted Services” means
the manufacture, distribution, wholesale and sale of Restricted Products, healthcare services and any other services that we or our subsidiaries
have provided or are researching, developing, performing and/or providing at any time during the two years immediately preceding the
date of termination, or which Mr. Patel has obtained any trade secret or other confidential information about at any time during the
two years immediately preceding the date of termination of the agreement. “Restricted Products” means pharmaceutical drugs
and other healthcare products and any other product, that we or our subsidiaries have provided or are researching, developing, manufacturing,
distributing, purchasing, selling and/or providing at any time during the two years immediately preceding the date the agreement is terminated,
or which Mr. Patel obtained any trade secret or other confidential information in connection with at any time during the two years immediately
preceding the date of termination of the agreement.
We
may terminate Mr. Patel’s employment (a) for “cause” (which is defined to include, a material breach of the agreement
by Mr. Patel, any act of misappropriation of funds or embezzlement by Mr. Patel, any act of fraud by Mr. Patel, or Mr. Patel being indicted
of, or pleading guilty or nolo contendere with respect to, theft, fraud, a crime involving moral turpitude, or a felony under federal
or applicable state law); (b) in the event Mr. Patel suffers a physical or mental disability which renders him unable to perform his
duties and obligations for either 90 consecutive days or 180 days in any 12-month period; (c) for any reason without “cause”;
or (d) upon expiration of the initial term of the agreement (or any renewal) upon notice as provided above. The agreement also automatically
terminates upon the death of Mr. Patel.
Mr.
Patel may terminate his employment (a) for “good reason” (i.e., (i) if his position or duties are modified to such an extent
that his duties are no longer consistent with the position of Chief Compliance Officer of the Company, (ii) there has been a material
breach by us of a material term of the agreement or Mr. Patel reasonably believes that we are violating any law which would have a material
adverse effect on our operations and such violation continues uncured thirty days after such breach and after notice thereof has been
provided to us by Mr. Patel, (iii) Mr. Patel’s compensation is reduced without his consent, or we fail to pay to Mr. Patel any
compensation due to him upon five days written notice from Mr. Patel informing us of such failure, or (iv) if Mr. Patel is also then
serving as a member of the Board and is not re-nominated by the Board to serve as a member of the Board at any annual meeting of stockholders
of the Company; provided, however, prior to any such termination by Mr. Patel for “good reason”, Mr. Patel must first advise
us in writing (within 15 days of the occurrence of such event) and provide us 15 days to cure (5 days in connection with the reduction
of Mr. Patel’s salary or the failure to pay amounts owed to him)); (b) for any reason without “good reason”; and (c)
upon expiration of the initial term of the agreement (or any renewal) upon notice as provided above.
In
the event that Mr. Patel’s employment is terminated for any reason (not including, however, a termination by us for “cause”
or a termination as a result of Mr. Patel’s death or disability) during the twelve month period following a Change of Control (a
“Change of Control Termination”) or in anticipation of a Change of Control, we are required to pay Mr. Patel, within 60 days
following the later of (i) the date of such Change of Control Termination; and (ii) the date of such Change of Control, a cash severance
payment in a lump sum in an amount equal to 3.0 times the sum of his current base salary and the amount of the last bonus payable to
Mr. Patel (the “Change of Control Payment”), which amount is due within 60 days of the later of (i) the date of such Change
of Control Termination; and (ii) the date of such Change of Control. If Mr. Patel’s employment terminates due to a Change of Control
Termination within six (6) months prior to a Change of Control, it will be deemed to be “in anticipation of a Change of Control”
for all purposes. In addition, in the event of a Change of Control, all of Mr. Patel’s equity-based compensation immediately vests
to Mr. Patel and any outstanding stock options held by Mr. Patel can be exercised by Mr. Patel until the earlier of (A) one (1) year
from the date of termination and (B) the latest date upon which such stock options would have expired by their original terms under any
circumstances, provided that if Mr. Patel’s employment ends in anticipation of a Change of Control and such equity-based compensation
awards or stock options have previously expired pursuant to their terms, the Company is required to pay Mr. Patel a lump sum payment,
payable on the same date as the Change of Control Payment, equal to the Black Scholes value of the expired and unexercised equity compensation
awards and stock options held by Mr. Patel on the date of termination, based on the value of such awards had they been exercisable through
the end of their stated term and had not previously expired. “Change of Control” for the purposes of the agreement means:
(a) any person obtaining beneficial ownership representing more than 50% of the total voting power represented by our then outstanding
voting securities without the approval of not fewer than two-thirds of our Board; (b) a merger or consolidation of us whether or not
approved by our Board, other than a merger or consolidation that would result in our voting securities immediately prior thereto continuing
to represent at least 50% of the total voting power outstanding immediately after such merger or consolidation, (c) our stockholders
approving a plan of complete liquidation or an agreement for the sale or disposition by us of all or substantially all of our assets,
or (d) as a result of the election of members to our Board, a majority of the Board consists of persons who are not members of the Board
on March 31, 2024, except in the event that such slate of directors is proposed by a committee of the Board or the Board; provided that
if the definition of “Change of Control” in our Stock Incentive Plans or Equity Compensation Plans is more favorable than
the definition above, then such definition shall be controlling.
If
Mr. Patel’s employment is terminated pursuant to his death, disability, the end of the initial term (or any renewal term), without
“good reason” by Mr. Patel, or by us for “cause”, Mr. Patel is entitled to all salary accrued through the termination
date and no other benefits other than as required under the terms of employee benefit plans in which Mr. Patel was participating as of
the termination date. Additionally, any unvested stock options or equity compensation held by Mr. Patel immediately terminate and are
forfeited (unless otherwise provided in the applicable award) and any previously vested stock options (or if applicable equity compensation)
are subject to the terms and conditions set forth in the applicable stock incentive plan or equity compensation plan, or award agreement,
as such may describe the rights and obligations upon termination of employment of Mr. Patel.
If
Mr. Patel’s employment is terminated by Mr. Patel for “good reason”, or by us without “cause”, Mr. Patel
is entitled to continue to receive the salary due pursuant to the terms of the agreement at the rate in effect upon the termination date
for eighteen (18) months, plus the pro rata amount of any discretionary bonus and performance bonus he would have been due for the following
eighteen (18) months (with any metrics being extrapolated based on the last four (4) full prior quarters of the Company’s operations
prior to termination). Additionally, unvested benefits (whether equity or cash benefits and bonuses) will vest immediately upon such
termination and any outstanding stock options previously granted to Mr. Patel will vest immediately upon such termination and will be
exercisable until the earlier of (A) one year from the date of termination and (B) the latest date upon which such stock options would
have expired by their original terms under any circumstances. Mr. Patel is also to receive, if he elects, continued health insurance
under COBRA, paid for by the Company, for eighteen (18) months following the termination date (subject to certain rights which reduce
such obligation if Mr. Patel is covered by health insurance with a substantially similar level of insurance as prior to the termination).
The
agreement contains standard assignment of inventions, indemnification and confidentiality provisions. Further, Mr. Patel is subject to
non-solicitation covenants during the term of the agreement.
Although
Mr. Patel will be prohibited from competing with us while he is employed with us, he will only be prohibited from competing for twelve
months after his employment with us ends pursuant to the agreement.
See
also “2023 Increased Officer Compensation” above.
DIRECTOR
COMPENSATION
Summary
Independent Director Compensation Table
The
following table provides information regarding all compensation awarded to, earned by or paid to each person who served as a non-executive
director of the Company for some portion or all of 2024. Other than as set forth in the table and described more fully below, the Company
did not pay any fees, make any equity or non-equity awards, or pay any other compensation, to its non-employee directors. All compensation
paid to its employee directors is set forth in the tables summarizing executive officer compensation above.
| Name | |
Fees
Earned or paid
in cash | | |
Stock Awards* | | |
Option Awards** | | |
All Other Compensation | | |
Total | |
| | |
| | |
| | |
| | |
| | |
| |
| Donald G.
Fell(1) | |
$ | 41,250 | | |
$ | 149,443 | | |
$ | - | | |
$ | - | | |
$ | 190,693 | |
| Shankar Hariharan(2) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Narasimhan Mani(3) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Mayur Doshi(4) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Subbarao Jayanthi(5) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Candice Beaumont (6) | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | | |
$ | - | |
| Jeff Newell(7) | |
$ | 26,250 | | |
$ | 163,736 | | |
$ | - | | |
$ | - | | |
$ | 189,986 | |
| Michael L. Peterson(8) | |
$ | 41,250 | | |
$ | 149,443 | | |
$ | - | | |
$ | - | | |
$ | 190,693 | |
*
Amounts in this column represent the aggregate grant date fair value of awards computed in accordance with Financial Accounting Standards
Board Accounting Standard Codification Topic 718. Such grant date fair value does not take into account any estimated forfeitures. The
assumptions used in calculating the grant date fair value of restricted shares and option awards are set forth in the Critical Accounting
Estimates as disclosed in our Consolidated Financial Statements for the year ended December 31, 2023. The amount reported in this column
reflects the accounting cost for these awards and does not correspond to the actual economic value that may be received by the director
upon the vesting of the restricted shares, the exercise of the stock options, or any sale of the underlying shares of common stock.
**
Amounts in this column represent the aggregate grant date fair value of awards computed in accordance with the Black-Scholes option pricing
model. The Black-Scholes model considers several variables and assumptions in estimating the fair value of stock-based awards. These
variables include the per share fair value of the underlying common stock, exercise price, expected term, risk-free interest rate, expected
annual dividend yield and the expected stock price volatility over the expected term. The Company estimates volatility by reference to
the historical volatilities of the Company. The risk-free interest rate is based on the yield available on U.S. Treasury zero-coupon
issues similar in duration to the expected term of the equity-settled award.
(1)
As of December 31, 2024, Mr. Fell had been awarded 3,345 vested stock options and 222,217 shares of vested common stock of
the Company.
(2)
Dr. Hariharan was appointed to the Board on July 25, 2024, in connection with the Company’s acquisition of Scienture LLC.
As of December 31, 2024. Dr. Hariharan did not receive any fees or other compensation for serving on the Board
during a portion of 2024.
(3)
Dr. Mani was appointed to the Board on July 25, 2024, in connection with the Company’s acquisition of Scienture LLC. As of
December 31, 2024. Dr. Mani did not receive any fees or other compensation for serving on the Board
during a portion of 2024.
(4)
Mr. Doshi was appointed to the Board on May 28, 2024. As of December 31, 2024. Mr. Doshi did not receive any fees or other compensation for serving on the Board
during a portion of 2024.
(5)
Mr. Jayanthi was appointed to the Board on June 17, 2024. As of December 31, 2024. Mr. Jayanthi did not receive any fees or other compensation for serving on
the Board during a portion of 2024.
(6)
Ms. Beaumont was appointed to the Board on July 31, 2023, and then resigned from the Board effective April 10, 2024. Ms. Beaumont
did not receive any fees or other compensation for serving on the Board during a portion of 2024.
(7)
As previously disclosed, Mr. Newell voluntarily resigned from the Board effective May 30, 2024. As of December 31, 2024, Mr. Newell
had been awarded 80,702 shares of vested common stock of the Company.
(8) As
previously disclosed, Mr. Peterson voluntarily resigned from the Board effective May 30, 2024. As of December 31, 2024, Mr. Peterson
had been awarded 12,131 in vested stock options and 246,685 in shares of vested common stock of the Company.
Independent
Director Compensation Policy
Each
independent member of the Board is to receive an annual grant of restricted common stock of the Company equal to $55,000 in value, on
April 1st of each year (or such date thereafter as the awards are approved by the Board), and valued on such same date, based on the
closing sales price on such date (or the first business day thereafter), which restricted stock awards will vest at the rate of 1/4th
of such awards over the following four calendar quarters, subject to such directors continued service to the Company.
The
Company has also entered into an indemnification agreement with each member of the Board.
2024
Independent Director Compensation
The Board approved the issuance of 36,163 shares of the Company’s
common stock to each of Mr. Fell and Mr. Peterson for services rendered to the Company during fiscal 2024, which shares were valued at
$149,443 each. The Board also approved the issuance of 38,526 shares of the Company’s common stock to Mr. Newell for services rendered
during fiscal 2024, which shares were valued at $163,736. The shares vest immediately on the grant date, subject to each applicable independent
director’s continued service to the Company.
All of these awards were issued under the Incentive Plan and all awards
were evidenced by Restricted Stock Grant Agreements.
Each
independent director also receives an annual cash retainer of $35,000.
CHANGE
IN AUDITOR
Dismissal
of MaloneBailey, LLP
On
September 14, 2023, the Company dismissed MaloneBailey, LLP (“MaloneBailey”) as its independent registered public accounting
firm to audit the Company’s financial statements, effective as of such date. The dismissal of MaloneBailey was approved by the
Audit Committee. MaloneBailey’s audit report on the Company’s financial statements for each of the fiscal years ended December
31, 2022 and 2021 did not contain an adverse opinion or a disclaimer of opinion, nor was it qualified or modified as to uncertainty,
audit scope, or accounting principles.
During
the Company’s two most recent fiscal years and the subsequent interim period through June 30, 2023, there were no (i) disagreements
(as defined in Item 304(a)(1)(iv) of Regulation S-K under the Exchange Act and the related instructions to that Item) with MaloneBailey
on any matter of accounting principles or practices, financial statement disclosure, or auditing scope or procedure, which disagreement,
if not resolved to the satisfaction of MaloneBailey would have caused it to make reference to the subject matter of the disagreement
in connection with its report, or (ii) “reportable events” as that term is defined in Item 304(a)(1)(v) of Regulation S-K
under the Exchange Act.
Engagement
of CM3 Advisory
On
September 14, 2023, the Company engaged CM3 Advisory as its new independent registered public accounting firm of the Company. The engagement
of CM3 Advisory was approved by Audit Committee.
During
the Company’s two most recent fiscal years and the subsequent interim period through June 30, 2023, neither the Company nor anyone
on its behalf consulted with CM3 Advisory regarding: (i) the application of accounting principles to a specified transaction, either
completed or proposed, (ii) the type of audit opinion that might be rendered on the Company’s financial statements, and neither
a written report nor oral advice was provided to the Company that CM3 Advisory concluded was an important factor considered by the Company
in reaching a decision as to an accounting, auditing or financial reporting issue, or (iii) any matter that was either the subject of
a disagreement (as defined in Item 304(a)(1)(iv) of Regulation S-K under the Exchange Act and the related instructions to that Item)
or a reportable event (as described in Item 304(a)(1)(v) of Regulation S-K under the Exchange Act).
LEGAL
MATTERS
The
validity of the securities offered by this prospectus will be passed upon by Dykema Gossett PLLC.
EXPERTS
The
consolidated financial statements of Scienture Holdings, Inc. at December 31, 2023 and the consolidated financial statements of Scienture
Holdings, Inc. at December 31, 2022 incorporated by reference in this prospectus have been audited by CM3 Advisory and MaloneBailey,
LLP, each an independent registered public accounting firm, as set forth in their reports thereon, appearing therein, and are included
in reliance upon such report given on the authority of such firms as experts in accounting and auditing.
The
financial statements of Scienture, LLC at December 31, 2023 and December 31, 2022 incorporated by reference in this prospectus have been
audited by Suri & Co., an independent registered public accounting firm, as set forth in its report thereon, appearing therein, and
are included in reliance upon such report given on the authority of such firm as an expert in accounting and auditing.
information
incorporated by reference
The
SEC allows us to “incorporate by reference” information that we file with them. Incorporation by reference allows us to disclose
important information to you by referring you to those other documents. The information incorporated by reference is an important part
of this prospectus, and information that we file later with the SEC will automatically update and supersede this information. We filed
a registration statement on Form S-1 under the Securities Act with the SEC with respect to the securities being offered pursuant to this
prospectus. You should refer to the registration statement, including the exhibits and schedules attached to the registration statement
and the information incorporated by reference, for further information about us and the securities being offered pursuant to this prospectus.
The documents we are incorporating by reference into this prospectus are:
| |
● |
our
Annual Report on Form 10-K for the fiscal year ended December 31, 2023, filed on April 22, 2024, as amended by our Amendment No.
1 to Form 10-K filed on May 3, 2024; |
| |
|
|
| |
● |
our
Quarterly Report on Form 10-Q for the period ended March 31, 2024, filed on June 26, 2024; |
| |
|
|
| |
● |
our
Quarterly Report on Form 10-Q for the period ended June 30, 2024, filed on August 9, 2024; |
| |
|
|
| |
● |
our
Quarterly Report on Form 10-Q for the period ended September 30, 2024, filed on November 6, 2024; |
| |
|
|
| |
● |
our
Definitive Information Statement on Schedule 14C filed on August 28, 2024; |
| |
|
|
| |
● |
our Definitive Information Statement on Schedule 14C
filed on December 30, 2024; and |
| |
|
|
| |
● |
our
Current Reports on Form 8-K (other than portions thereof furnished under Item 2.02 or Item 7.01 of Form 8-K and exhibits accompanying
such reports that relate to such items) filed on January
17, 2024; February
16, 2024; March
6, 2024; May
23, 2024; May
30, 2024; June
20, 2024; and July
9, 2024; July
31, 2024; September
24, 2024; October
8, 2024; October
11, 2024; and November 26, 2024. |
We
also incorporate by reference into this prospectus all documents (other than current reports furnished under Item 2.02 or Item 7.01 of
Form 8-K and exhibits filed on such form that are related to such items) that are filed by us with the SEC pursuant to Sections 13(a),
13(c), 14 or 15(d) of the Exchange Act after the date of the initial registration statement of which this prospectus is a part and prior
to the effectiveness of such registration statement and all documents that are filed by us with the SEC pursuant to Sections 13(a), 13(c),
14 or 15(d) of the Exchange Act after the date of this prospectus but prior to the termination of the offering. These documents include
periodic reports, such as Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K, as well as proxy
statements and information statements.
Any
statement contained herein or in a document incorporated or deemed to be incorporated by reference into this prospectus will be deemed
to be modified or superseded for purposes of the document to the extent that a statement contained in this prospectus or any other subsequently
filed document that is deemed to be incorporated by reference into this document modifies or supersedes the statement.
We
will provide without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral
request, a copy of any or all documents that are incorporated by reference into this prospectus, but not delivered with the prospectus,
other than exhibits to such documents unless such exhibits are specifically incorporated by reference into the documents that this prospectus
incorporates. You should direct oral or written requests to our corporate secretary, who can be contacted at 6308 Benjamin Rd, Suite
708, Tampa, Florida 33634 or (800) 261-0281. You may also access these documents, free of charge on the SEC’s website at www.sec.gov.
WHERE
YOU CAN FIND MORE INFORMATION
We
have filed with the SEC a registration statement on Form S-1, which includes amendments and exhibits, under the Securities Act and the
rules and regulations under the Securities Act for the registration of the common stock being offered by this prospectus. This prospectus,
which constitutes a part of the registration statement, does not contain all the information that is in the registration statement and
its exhibits and schedules. Statements in this prospectus that summarize documents are not necessarily complete, and in each case you
should refer to the copy of the document filed as an exhibit to the registration statement. The registration statement and other public
filings can be obtained from the SEC’s internet site at www.sec.gov.
We
file annual, quarterly, and current reports, proxy statements and other information with the SEC. Our SEC filings are available to the
public over the Internet at the SEC’s website at www.sec.gov. Copies of documents filed by us with the SEC are also available
from us without charge, upon oral or written request to our Secretary, who can be contacted at 6308 Benjamin Rd, Suite 708, Tampa, Florida
33634 or (800) 261-0281. Our website addresses is https://scienture.com. Information on, or that may be accessed through, our
website is not incorporated by reference into this prospectus and should not be considered a part of this prospectus.
INDEX
TO FINANCIAL INFORMATION
Scienture,
LLC (FKA Scienture, Inc.) Financial Statements
Scienture
Holdings, Inc. (FKA TRxADE HEALTH, Inc.) Unaudited Pro Forma Financial Statements
Scienture
Inc.
Balance
Sheets
Unaudited
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Assets | |
| | | |
| | |
| Current Assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 114,210 | | |
$ | 1,123,878 | |
| Accounts receivable | |
| - | | |
| 66,414 | |
| Other receivables | |
| 485 | | |
| 485 | |
| Total Current Assets | |
| 114,695 | | |
| 1,190,777 | |
| Operating lease, right of use asset | |
| 61,579 | | |
| 64,091 | |
| Total Assets | |
$ | 176,274 | | |
$ | 1,254,868 | |
| | |
| | | |
| | |
| Liabilities and Stockholders’ Equity | |
| | | |
| | |
| Current Liabilities: | |
| | | |
| | |
| Accounts payable | |
$ | 884,581 | | |
$ | 107,175 | |
| Accrued expenses and other liabilities | |
| 1,198,822 | | |
| 332,211 | |
| Convertible notes | |
| - | | |
| 3,665,220 | |
| Operating lease liability | |
| 22,567 | | |
| 21,404 | |
| Total Current Liabilities | |
| 2,105,970 | | |
| 4,126,010 | |
| Long-term convertible notes, net of debt discount | |
| 1,734,661 | | |
| 1,625,117 | |
| Operating Lease Liability, non current | |
| 39,319 | | |
| 42,893 | |
| Development agreement liability | |
| 1,285,000 | | |
| - | |
| Total Liabilities | |
| 5,164,950 | | |
| 5,794,020 | |
| | |
| | | |
| | |
| Commitments and contingencies (Refer Note 8) | |
| - | | |
| - | |
| Stockholders’ Deficit: | |
| | | |
| | |
| Preferred stock, $.0001
par value, 3,365,657
and 2,400,000 authorized, issued and outstanding as of June 30, 2024, and December 31, 2023, respectively | |
| 337 | | |
| 240 | |
| Common stock, $0.0001
par value, 10,000,000
authorized, 5,000,000
issued and outstanding as of both June 30, 2024, and December 31, 2023 | |
| 500 | | |
| 500 | |
| Additional paid-in capital | |
| 10,835,257 | | |
| 6,849,064 | |
| Accumulated deficit | |
| (15,824,770 | ) | |
| (11,388,956 | ) |
| Total stockholders’ deficit | |
| (4,988,676 | ) | |
| (4,539,152 | ) |
| Total Liabilities and Stockholders’ Deficit | |
$ | 176,274 | | |
$ | 1,254,868 | |
Scienture
Inc.
Statements
of Operations and Comprehensive Loss
Unaudited
| | |
2024 | | |
2023 | |
| | |
Six
Months Ended June 30, | |
| | |
2024 | | |
2023 | |
| Revenue | |
$ | - | | |
$ | 800,000 | |
| | |
| | | |
| | |
| Operating Expenses: | |
| | | |
| | |
| Research and development | |
| 1,520,947 | | |
| 946,435 | |
| General and administrative | |
| 1,413,893 | | |
| 267,236 | |
| Termination fee | |
| 1,285,000 | | |
| - | |
| Total operating expenses | |
| 4,219,840 | | |
| 1,213,671 | |
| | |
| | | |
| | |
| Loss from Operations | |
| (4,219,840 | ) | |
| (413,671 | ) |
| | |
| | | |
| | |
| Other Income (Expense) | |
| | | |
| | |
| Dividend income | |
| | | |
| | |
| Other income | |
| 11,931 | | |
| 18,304 | |
| Interest expense | |
| (227,905 | ) | |
| (76,203 | ) |
| Miscellaneous income | |
| | | |
| | |
| Total other expense | |
| (215,974 | ) | |
| (57,899 | ) |
| | |
| | | |
| | |
| Net Loss | |
$ | (4,435,814 | ) | |
$ | (471,570 | ) |
| | |
| | | |
| | |
| Net loss per share - basic and diluted | |
$ | (0.89 | ) | |
$ | (0.09 | ) |
| Weighted-average shares used to compute net loss per share
- diluted | |
| 5,000,000 | | |
| 5,000,000 | |
Scienture
Inc.
Statements
of Stockholders’ Deficit
Unaudited
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| | |
Preferred
Stock | | |
Common
Stock | | |
Additional
Paid-In | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance at December 31, 2021 | |
| 2,400,000 | | |
$ | 240 | | |
| 4,850,000 | | |
$ | 485 | | |
$ | 6,111,783 | | |
$ | (5,439,589 | ) | |
$ | 672,919 | |
| Common stock issued for services | |
| - | | |
| - | | |
| 150,000 | | |
| 15 | | |
| 145,485 | | |
| - | | |
| 145,500 | |
| Stock-based compensation expenses | |
| - | | |
| - | | |
| - | | |
| - | | |
| 68,087 | | |
| - | | |
| 68,087 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,708,378 | ) | |
| (3,708,378 | ) |
| Balance at December 31, 2022 | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000 | | |
$ | 500 | | |
$ | 6,325,355 | | |
$ | (9,147,967 | ) | |
$ | (2,821,872 | ) |
| Stock-based compensation expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| 39,724 | | |
| - | | |
| 39,724 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (471,570 | ) | |
| (471,570 | ) |
| Balance at June 30, 2023 | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000 | | |
$ | 500 | | |
$ | 6,365,079 | | |
$ | (9,619,537 | ) | |
$ | (3,253,718 | ) |
| | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | | |
| | |
| Balance at December 31, 2023 | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000 | | |
$ | 500 | | |
$ | 6,849,064 | | |
$ | (11,388,956 | ) | |
$ | (4,539,152 | ) |
| Conversion of notes into preferred stock | |
| 965,657 | | |
| 97 | | |
| - | | |
| - | | |
| 3,941,356 | | |
| - | | |
| 3,941,453 | |
| Stock-based compensation expense | |
| - | | |
| - | | |
| - | | |
| - | | |
| 44,837 | | |
| - | | |
| 44,837 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (4,435,814 | ) | |
| (4,435,814 | ) |
| Balance at June 30, 2024 | |
| 3,365,657 | | |
$ | 337 | | |
| 5,000,000 | | |
$ | 500 | | |
$ | 10,835,257 | | |
$ | (15,824,770 | ) | |
$ | (4,988,676 | ) |
Scienture
Inc.
Statements
of Cash Flows
Unaudited
| | |
2024 | | |
2023 | |
| | |
Six
Months Ended June 30, | |
| | |
2024 | | |
2023 | |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (4,435,814 | ) | |
$ | (471,570 | ) |
| Adjustments to reconcile Net loss to net cash used in operating
activities: | |
| | | |
| | |
| Common stock issued for services | |
| | | |
| | |
| Amortization of debt discount | |
| 109,544 | | |
| - | |
| Stock-based compensation expense | |
| 44,837 | | |
| 39,724 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts receivable | |
| 66,414 | | |
| (300,000 | ) |
| Accounts payable | |
| 777,406 | | |
| 29,729 | |
| Accrued expenses and other liabilities | |
| 1,142,843 | | |
| 76,212 | |
| Development agreement liability | |
| 1,285,000 | | |
| - | |
| Operating lease liability, Net | |
| 102 | | |
| - | |
| Net cash used in operating activities | |
| (1,009,668 | ) | |
| (625,905 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from the issuance of convertible notes | |
| | | |
| 400,000 | |
| Proceeds from the issuance of long-term convertible debt | |
| | | |
| | |
| Net cash used in financing activities | |
| - | | |
| 400,000 | |
| | |
| | | |
| | |
| Net change in cash and cash equivalents | |
| (1,009,668 | ) | |
| (225,905 | ) |
| Cash and cash equivalents at beginning of period | |
| 1,123,878 | | |
| 604,813 | |
| Cash and cash equivalents at end of period | |
$ | 114,210 | | |
$ | 378,908 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | - | | |
$ | - | |
| Supplemental disclosure of non-cash financing activities: | |
| | | |
| | |
| Conversion of notes and accrued interest into preferred
stock | |
$ | 3,941,453 | | |
$ | - | |
| Warrants issued in connection with long-term convertible
debt | |
| | | |
| | |
Note
1 Organization Overview and Basis of Presentation
Nature
of Operations
Scienture
Inc. (“the Company”) is a pharmaceutical research company which is engaged in the research and development of branded pharmaceutical
products. The IP application process of the company initiated in November 2019 and commenced the product development activities from
January 2020. The Company also plans to foray into commercialization of innovative and branded pharmaceutical products in the US market.
The
Company was incorporated in the state of Delaware in June 2019. The Company is headquartered in Hauppauge, New York, United States of
America.
Basis
of Presentation
The
Company’s fiscal year ends on December 31.
The
accompanying financial statements for the periods ending June 30, 2024 and 2023 have been prepared in accordance with accounting principles
generally accepted in the United States (“U.S.GAAP”).
Note
2 Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S.GAAP requires the Company to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. These estimates are based on information available through the
date of the issuance of the financial statements and actual results could differ from those estimates. To the extent there are material
differences between the Company’s estimates and the actual results, the Company’s future consolidated results of operation
may be affected. Areas requiring significant estimates and assumptions by the Company include, but are not limited to:
| |
● |
fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| |
● |
accruals
for estimated liabilities; |
| |
● |
the
valuation of stock-based compensation awards ; and |
| |
● |
provisions
for income taxes and related valuation allowances and tax uncertainties. |
Unaudited
Interim Financial Information
The
unaudited condensed consolidated interim financial statements and related notes have been prepared in accordance with U.S. GAAP for interim
financial information, within the rules and regulations of the United States Securities and Exchange Commission (the “SEC”).
Certain information and disclosures normally included in the annual financial statements prepared in accordance with U.S. GAAP have been
condensed or omitted pursuant to such rules and regulations. The unaudited interim financial statements have been prepared on a basis
consistent with the audited financial statements and in the opinion of management, reflect all adjustments, consisting of only normal
recurring adjustments, necessary for the fair presentation of the results for the interim periods presented and of the financial condition
as of the date of the interim balance sheet. The financial data and the other information disclosed in these notes to the interim financial
statements related to the six-month periods are unaudited. Unaudited interim results are not necessarily indicative of the results for
the full fiscal year.
The
accompanying unaudited interim condensed consolidated financial statements should be read in conjunction with the Company’s audited
financial statements and the notes thereto for the year ended December 31, 2023.
Liquidity
The
entity has just commenced operations and is expected to be funded by the stockholders for liquidity purposes. The liquidity position
of the entity is also dependent on the fundings by the additional development partners.
Comprehensive
Loss
Comprehensive
loss includes net loss as well as other changes in stockholder’s equity that result from transactions and economic events other
than those with stockholders. There was no difference between net loss and comprehensive loss presented in the financial statements for
the six months ended June 30, 2024 and 2023.
Segment
Reporting
The
Company’s chief operating decision-maker is its Chief Executive Officer, who makes resource allocation decisions and assesses performance
based on financial information presented on an aggregate basis. There are no segment managers who are held accountable by the chief operating
decision-maker, or anyone else, for any planning, strategy and key decision-making regarding operations. Accordingly, the Company has
a single reportable segment and operating segment structure.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash
equivalents. Cash equivalents consist of amounts invested in money market funds and are stated at fair value.
Accounts
Receivable
Accounts
receivable consist of milestone payments due from development partners as a consideration for the rights granted for the commercialization
of the products to be developed. The Company reviews its accounts receivable and provides allowances of specific amounts if collectability
is no longer reasonably assured based on historical experience and specific collection issues. The allowance for doubtful accounts was
$0 as of June 30, 2024 and December 31, 2023, respectively.
Revenue
Recognition
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services.
The
Company adopted FASB ASU No. 2014-09, Revenue from Contracts with Customers and the related amendments, which are codified into ASC 606,
which establishes a broad principle that requires entities to assess the products or services promised in contracts with customers at
contract inception to determine the appropriate unit at which to record revenues, which is referred to as a performance obligation. Revenue
is recognized when control of the promised products or services is transferred to customers, at an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those products or services.
To
determine revenue recognition for arrangements that the Company determines are within the scope of ASC 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii)
determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it
is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the
customer. At contract inception, once the contract was determined to be within the scope of ASC 606, the Company assessed the goods or
services promised within each contract and determined those that were performance obligations, and assessed whether each promised good
or service was distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective
performance obligation when (or as) the performance obligation is satisfied.
A
performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in
ASC 606. The Company recognizes revenue at the point of sale of service.
Exclusive
License and Commercial Agreements
The
Company entered into an exclusive license and commercial agreement with Kesin Pharma Corporation, a related party where the Company granted
the exclusive license rights to commercialize SCN-102 in 2022 and SCN-104 in 2023 to Kesin (SCN-102 and SCN-104 are together referred
to as “the Products”) for use in the United States of America. In consideration of the rights granted, the Company is in
receipt of milestone payments and reimbursement of costs actually incurred related to the products. Revenue has been recognized when
such development milestone events take place and the amounts are due to be received. The Company recognized $800,000 for the six months
ended June 30, 2023, at the point when the development milestone events occurred.
In
March 2024, the parties have terminated the agreement, and the parties agreed that, Scienture shall pay Kesin a total gross amount of
$1,285,000 upon commercialization of product via a royalty arrangement.
This
agreement also requires that if the full $1,285,00 has not been repaid within two years of the early of i) commercial launch or ii) 120
from FDA approval, then interest will accrue prospectively at a rate of 8% annually on unpaid balance. Accordingly, the Company recorded
a $1,285,000 termination fee liability. As of the date of issue of financial statements, the entire amount is outstanding.
Fair
Value of Financial Instruments
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes
the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available.
Observable inputs are inputs that market participants would use in pricing the asset or liability and are developed based on market data
obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions of what
market participants would use in pricing the asset or liability based on the best information available in the circumstances. The financial
and nonfinancial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurement.
The hierarchy is presented down into three levels based on the reliability of the inputs.
| |
Level
1 |
Quoted
prices are available in active markets for identical assets or liabilities. |
| |
|
|
| |
Level
2 |
Observable
inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets
or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially
the full term of the assets or liabilities. |
| |
|
|
| |
Level
3 |
Unobservable
pricing inputs that are generally less observable from objective sources, such as discounted cash flow models or valuations. |
The
carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term convertible notes approximate their
fair value because of the short-term nature of these instruments. The carrying amount of long-term convertible debt approximate the fair
value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Concentration
of Credit Risks and Major Customers
Financial
instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The
Company places its cash and cash equivalents with financial institutions. During the six months ended June 30, 2024, the Company had
one development partner that accounted for the entire revenue recognized in the Statement of Comprehensive Loss.
Research
& Development Expenses
Research
and development costs are expensed in the period incurred in accordance with ASC 730. Research and development expenses consist of independent
contractor costs , costs for outsourced analytical research and development activities, batch manufacturing cost and, advisory costs
as a part of research, market research costs and other regulatory consulting costs.
Stock-Based
Compensation
The
Company’s stock-based compensation expense relates to stock options. Stock-based compensation expense for its stock-based awards
is based on their grant date fair value. The Company estimates the fair value of stock option awards on the grant date using the Black-Scholes
option-pricing model. The Black-Scholes model considers several variables and assumptions in estimating the fair value of stock-based
awards. These variables include the per share fair value of the underlying common stock, exercise price, expected term, risk-free interest
rate, expected annual dividend yield and the expected stock price volatility over the expected term. The Company has estimated volatility
by reference to the historical volatilities of the Company and that of similar publicly traded peer companies. The risk-free interest
rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the equity-settled
award.
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
The Company accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
Net
Loss per share
Basic
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period,
adjusted for outstanding shares that are subject to repurchase.
For
the calculation of diluted net loss per share, basic net loss per share is adjusted by the effect of dilutive securities if any. Diluted
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding and potential
common stock outstanding, if dilutive. For periods in which the Company reports net losses, diluted net loss per share is the same as
basic net loss per share because potentially dilutive shares of common stock are not assumed to have been issued if their effect is anti-dilutive.
Disclosure - Schedule of Deferred Tax Assets and Related Valuation Allowance (Details)
Accounting
Pronouncements Not Yet Adopted
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2023-07, “Segment
Reporting (Topic 280): Improvements to Reportable Segment Disclosures” (“ASU 2023-07”), which requires additional operating
segment disclosures in annual and interim consolidated financial statements. ASU 2023-07 is effective for annual periods beginning after
December 15, 2023 and for interim periods beginning after December 15, 2024 on a retrospective basis, with early adoption permitted.
The Company is evaluating the effect of adopting ASU 2023-07.
In
December 2023, the FASB issued Accounting Standards Update No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”
(“ASU 2023-09”), which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components
of the effective tax rate reconciliation and modifies other income tax-related disclosures. ASU 2023-09 is effective for annual periods
beginning after December 15, 2024 on a retrospective or prospective basis. The Company is evaluating the effect of adopting ASU 2023-09.
Note
3 Going Concern
The
Company has a net loss of ($4,435,814) for the six months ended June 30, 2024 and stockholders’ deficit of ($4,988,676) as of June
30, 2024. The Company’s situation raises a substantial doubt on whether the entity can continue as a going concern in the next
twelve months.
The
Company’s ability to continue as a going concern in the next twelve months following the date the financial statements were available
to be issued is dependent upon its ability to produce revenues and/or obtain financing sufficient to meet current and future obligations
and deploy such to produce profitable operating results.
Management
has evaluated these conditions and plans to generate revenues and raise capital as needed to satisfy its capital needs. During the next
twelve months, the Company intends to fund its operations through debt and/or equity financing.
There
are no assurances that management will be able to raise capital on terms acceptable to the Company. If it is unable to obtain sufficient
amount of additional capital, it may be required to reduce the scope of its planned development, which could harm its business, financial
condition, and operating results. The accompanying financial statements do not include any adjustments that might result from these uncertainties.
The
Company has evaluated whether there are certain conditions and events, considered in the aggregate, that raise substantial doubt about
the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
Note
4 Cash and Cash Equivalents
Cash
and cash equivalents consist of the following:
Schedule
of Cash and Cash Equivalents
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Balances with banks | |
$ | 116,107 | | |
$ | 31,943 | |
| Money market securities(Highly liquid investments) | |
| - | | |
| 1,091,935 | |
| Total Cash and Cash Equivalents | |
$ | 116,106 | | |
$ | 1,123,878 | |
Money
market securities were considered a Level 1 financial instrument.
Note
5 Convertible Notes
The
carrying value of the convertible notes approximate their fair value because of the short-term nature of these instruments. The convertible
notes issued bear an interest at a rate of 8% per annum and certain notes issued prior to 2022 bear an interest at a rate of 2% per annum.
As of December 31, 2023, there $3,665,220 in outstanding principal. All the short-term convertible notes matured during the period of
December 2023. In March 2024, the Company had converted the outstanding principal of $3,665,220 and the accrued interest through the
date of conversion amounting to $276,233 into an aggregate of 965,567 shares of preferred stock of the Company.
Note
6 Long-Term Convertible Debt, net of debt discount
In
September 2023, the Company entered into a loan agreement with NVK Finance LLC, a Nebraska Limited Liability Company (‘NVK”)
for $2,000,000. The Board Member of the Company has significant influence in the decision making in NVK and hence considered as a related
party. The debt shall accrue interest at a per annum rate equal to Prime Rate plus 7 percent and the prime rates shall be adjusted quarterly
commencing on December 2023. As of June 30, 2024 and December 31, 2023, the interest rate was 15.50%. The debt is collateralized by all
of the Company’s receivables, cash and cash equivalents and the title in Intellectual Property Rights and all proceeds thereof.
The principal is entirely repayable on the maturity date i.e. September 2025 and interest shall be paid monthly upon a Qualified Financing
as defined in the Loan Agreement. Interest expense related to the debt amounted to $95,583 for the year ended December 31, 2023 and the
principal amount is entirely outstanding as at December 31,2023. The outstanding balance under the NVK debt is convertible into common
stock of the Company at a fully-diluted Company valuation of $60,000,000.
In
connection with the NVK debt, the Company granted 509,014 warrants to purchase common stock. The fair value of the warrants was $444,260
using Black-Scholes option pricing model, which will be amortized to interest expense over the life of the notes. During the six months
ended June 30, 2024, the Company amortized $109,544 of the debt discount to interest expense.
Long-term
convertible debt, net of debt discount, consisted of the following:
Schedule of Long Term Convertible
Debt Net of Discount
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Principal | |
$ | 2,000,000 | | |
$ | 2,000,000 | |
| Less: Unamortized debt discount | |
| 265,339 | | |
| 374,883 | |
| Long-term convertible notes, net of debt discount | |
$ | 1,734,661 | | |
$ | 1,625,117 | |
Maturities
of the outstanding notes are as follows:
Schedule of Maturities Notes
| Years Ending December 31 | |
| |
| 2024 | |
$ | - | |
| Year 1 | |
$ | - | |
| 2025 | |
| 2,000,000 | |
| Year 3 | |
| | |
| Total | |
$ | 2,000,000 | |
Note
7 Fair Value of Financial Instruments
The
carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and short-term convertible notes
approximate their fair value because of the short-term nature of these instruments. The carrying amount of long-term debt approximates
fair value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Note
8 Commitment and Contingencies
The
Company, in conjunction with its legal counsel, assesses the need to record a liability for litigation or loss contingencies. A liability
is recorded when and if it is determined that such a liability for litigation or loss contingencies is both probable and estimable. The
Company does not record any anticipated gains relating to its litigation or legal claims. The gains are only recorded upon receipt of
the settlement.
Although
the results of legal proceedings and claims cannot be predicted with certainty, the Company is not currently a party to any legal proceedings,
which would, individually or in the aggregate, have a material adverse effect on its results of operations, cash flows, or financial
position.
In
August 2024, Kesin demanded immediate payment of the full amount under the Kesin Termination Agreement, alleging the full amount is payable
in connection with the consummation Scienture’s business combination with the Company. Scienture has disputed that the amount is
now payable, and the parties are in discussions to resolve the issue. There can be no assurance that an amicable resolution will be obtained.
If Kesin brings a legal action, Scienture will vigorously defend it.
Note
9 Related Party Transactions
The
Company had entered into an Exclusive and Commercial agreement with Kesin Pharma Corporation (“Kesin”), in which one of the
company’s board member is the President and CEO, and had a significant influence in the decision making, which makes it a related
party. Sales made to Kesin as a part of milestone structure for the six months ended June 30, 2024 and 2023 were $0 and $800,000, respectively.
The Company terminated the agreement with Kesin in March 2024, and recorded a termination fee and related liability of $1,285,000 as
of June 30, 2024.
The
Company has leased its office from Saptalis Pharmaceuticals LLC (“Saptalis”) , in which one of the Company’s Director
is the President and CEO. Lease payments made during the six months ended June 30, 2024 and 2023 are $14,440 and $0, respectively. The
Company has also engaged Saptalis to provide development services and conduct testing and studies for the products under development
by the Company. Expenses incurred towards such testing and studies which is included in the Research and Development Expenses in the
Statement of Comprehensive Loss for the six months ended June 30, 2024 and 2023 amounted to $106,539 and $189,027, respectively.
During
the six months ended June 30, 2023, a related party to a director issued a convertible note to the Company for $250,000.
In
July 2024, officers of the company provided a short term promissory note to the Company for $265,000
Schedule of Relatives or Related Parties to Directors Purchased Securities
Note
10 Scienture Inc. 2020 Stock Option and Grant Plan
The
Stock Option and Grant Plan allows for the issuance of incentive stock options (“ISOs”), non-statutory stock options (“NSOs”).
ISOs may be granted only to the Company’s employees (including officers and directors who are also considered employees) and ex-employees.
NSOs may be granted to the Company’s employees and service providers such as advisors etc. Options under the Stock Option and Grant
Plan have a contractual term of not more than 10 years.
A
summary of the Company’s stock option activity under the Plans is as follows:
Summary of Stock Option Activity
| | |
Outstanding
Options | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Remaining
Term
(Years) | |
| Balance as of December 31, 2023 | |
| 655,000 | | |
$ | 0.90 | | |
| 7.56 | |
| Granted | |
| 142,199 | | |
| 1.13 | | |
| | |
| Exercised | |
| - | | |
| - | | |
| | |
| Cancelled and forfeited | |
| - | | |
| - | | |
| | |
| Balance as of June 20, 2024 | |
| 797,199 | | |
$ | 0.94 | | |
| 6.21 | |
| | |
| | | |
| | | |
| | |
| Vested and exercisable as of June 30, 2024 | |
| 499,089 | | |
$ | 0.84 | | |
| 5.62 | |
The
weighted-average grant date fair value of options granted during the six months ended June 30, 2024 was $0.76 per share.
The
Company recorded stock-based compensation expense in the Statement of Operations and Comprehensive Loss for the periods presented as
follows:
Schedule of Stock-based Compensation Expense
| | |
2024 | | |
2023 | |
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| General and Administrative Expenses | |
$ | 44,837 | | |
$ | 39,724 | |
| Total stock-based compensation expense | |
$ | 44,837 | | |
$ | 39,724 | |
Stock
Option Valuation Assumptions
The
fair value of each employee option grant was estimated on the date of grant using the Black-Scholes option pricing model and the following
assumptions for the periods indicated:
Schedule of Stock Option Valuation Assumptions
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Expected volatility | |
| 72 | % | |
| 65%
- 75 | % |
| Risk-free interest rate | |
| 4.1%
- 4.4 | % | |
| 0.5%
- 3.5 | % |
| Expected term | |
| 5.7
- 6.1 years | | |
| 5.9
- 6.0 years | |
| Expected dividend | |
| 0 | % | |
| 0 | % |
Note
11 Warrants
As
of June 30, 2024, there were 509,014 warrants outstanding and exercisable with an exercise price of $0.01 per share. The warrants were
granted in connection with the NVK debt (Refer - Note 6 - Long-Term Convertible Debt, net of debt discount).
Summary of Assumptions Used to Estimate Fair Value of Warrants
Note
12 Net Loss per Share
Stock
options to purchase 799,199 and 655,000 shares of common stock, warrants to purchase 509,014 and 0 common stock, convertible preferred
stock and convertible notes to purchase 3,365,669 and 3,195,911 common stock and long-term convertible debt to purchase 0 and 3,350,000
shares common stock were outstanding at June 30, 2024 and 2023, respectively, that were not included in the computation of diluted weighted
average common shares outstanding because their effect would have been anti-dilutive.
Note
13 Leases
The
Company determines if an arrangement is a lease at inception. Operating leases are included in Operating lease, Right of Use asset, Operating
Lease Liability (Current and Non-Current) in the Company’s balance sheets. The ROU assets represent the Company’s right to
use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising
from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments
over the lease term. As most of the leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate
at commencement date. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain
that the Company will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
As a practical expedient, the Company elected, for all office and facility leases, not to separate non-lease components from lease components
and instead to account for each separate lease component and its associated non-lease components as a single lease component. The Company
made an accounting policy election by class of underlying asset not to recognize the lease liability and related right-of-use asset for
leases with a term of one year or less.
The
Company has an operating lease for administrative office. The lease has remaining lease term around three years.
The
components of lease expense were as follows:
Schedule
of Lease Expense
| | |
| | | |
| | |
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Operating lease costs | |
| | | |
| | |
| Amortization of ROU Assets | |
$ | 2,152 | | |
$ | - | |
| Interest on Lease Liabilities | |
$ | 1,190 | | |
$ | - | |
| Short term lease costs | |
$ | 17,010 | | |
$ | - | |
Supplemental
balance sheet information related to leases was as follows:
Schedule
of Supplemental Balance Sheet Information Related To Leases
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Operating Leases | |
| | | |
| | |
| Right of Use Assets | |
$ | 61,579 | | |
$ | 64,091 | |
| | |
| | | |
| | |
| Short term Lease liabilities | |
$ | 22,567 | | |
$ | 21,404 | |
| Long term Lease liabilities | |
$ | 39,319 | | |
$ | 42,893 | |
| Total Lease Liabilities | |
$ | 61,886 | | |
$ | 64,297 | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term (in years) | |
| 2.33 | | |
| 2.83 | |
| | |
| | | |
| | |
| Weighted Average Discount Rate | |
| 15.50 | % | |
| 15.50 | % |
Note
14 Subsequent Events
In
July 2024, the executives of the Company issued a short-term loan to Company for an aggregate amount of $250,000.
In
July 2024, all of the unvested options per the Company’s Stock Option and Grant Plan became vested.
Business
Combination
On
July 25, 2024, Scienture, Inc. (the “Company”) entered into and closed an Agreement and Plan of Merger (the “Merger
Agreement”) with MEDS, MEDS Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of MEDS (“Merger Sub I”)
and MEDS Merger Sub II, LLC, a Delaware limited liability company and wholly owned subsidiary of the Company (“Merger Sub II”).
Pursuant to the Merger Agreement, (i) Merger Sub I merged with and into the Company, with the Company continuing as the surviving entity
and a wholly owned subsidiary of MEDS, and (ii) the Company merged with and into Merger Sub II, with Merger Sub II continuing as the
surviving entity.
Management
has evaluated subsequent events through August 15, 2024, the date the financial statements were available to be issued.
Report
of Independent Registered Public Accounting Firm
To
the Stockholders and Board of Directors of Scienture Inc.
Opinion
on the Financial Statements
We
have audited the accompanying balance sheets of Scienture Inc. (the “Company”) as of December 31, 2023 and 2022, the related
statements of operations and comprehensive loss, statements of stockholders’ deficit and statements of cash flows, and the related
notes collectively referred to as the “financial statements” for each of the two years in the period ended December 31, 2023.
In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December
31, 2023 and 2022, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2023,
in conformity with Generally Accepted Accounting Principles of United States of America. We were appointed as the independent auditors
of Scienture Inc. since 2022.
Matters
related to Going Concern
The
accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note
3 to the financial statements, if the Company is unable to raise additional funds to alleviate liquidity needs, it may be required to
reduce the scope of its planned development. The company has suffered losses from operations and has a net capital deficiency that raise
substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described
in Note 3 to the financial statements. The financial statements do not include any adjustments that might result from the outcome of
this uncertainty.
Responsibilities
of the Management for the Financial Statements
These
financial statements are the responsibility of the Company’s management. In preparing the financial statements, management is required
to evaluate whether there are conditions or events, considered in the aggregate, that raise substantial doubt about the Company’s
ability to continue as a going concern for one year after the date that the financial statements are available to be issued. Our responsibility
is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered
with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect
to the Company in accordance with the applicable rules and regulations of PCAOB.
We
conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain
reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The company
is not required to have, nor we have engaged to perform, an audit of its internal control over financial reporting. As part of our audit,
we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion
on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our
audit included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or
fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding
the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant
estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audit provides
a reasonable basis for our opinion.
Critical
Audit Matter
Critical
audit matters are matters arising from the current period audit of the financial statements that were communicated or required to be
communicated to the audit committee and that:
| (1) |
relate
to accounts or disclosures that are material to the financial statements and |
| |
|
| (2)
|
involved
our especially challenging, subjective, or complex judgments. |
We
determined that there are no critical audit matters.
We
have served as the Company’s auditors since 2022.
/s/
Suri & Co., Chartered Accountants
Date:
July 31,2024
Place:
Chennai, India
Scienture
Inc.
Balance
Sheets
| | |
| | |
| |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Assets | |
| | |
| |
| Current Assets: | |
| | | |
| | |
| Cash and cash equivalents | |
$ | 1,123,878 | | |
$ | 604,813 | |
| Accounts receivable | |
| 66,414 | | |
| - | |
| Other receivables | |
| 485 | | |
| 485 | |
| Total Current Assets | |
| 1,190,777 | | |
| 605,298 | |
| Operating lease, right of use asset | |
| 64,091 | | |
| - | |
| Total Assets | |
$ | 1,254,868 | | |
$ | 605,298 | |
| | |
| | | |
| | |
| Liabilities and Stockholders’ Deficit | |
| | | |
| | |
| Current Liabilities: | |
| | | |
| | |
| Accounts payable | |
| 107,175 | | |
| 393,676 | |
| Accrued expenses and other liabilities | |
| 332,211 | | |
| 83,494 | |
| Convertible notes | |
| 3,665,220 | | |
| 2,950,000 | |
| Operating lease liability | |
| 21,404 | | |
| - | |
| Total Current Liabilities | |
| 4,126,010 | | |
| 3,427,170 | |
| Long-term convertible debt, net of debt discount | |
| 1,625,117 | | |
| - | |
| Operating lease liability, non current | |
| 42,893 | | |
| - | |
| Total Liabilities | |
| 5,794,020 | | |
| 3,427,170 | |
| | |
| | | |
| | |
| Commitments and contingencies (Refer Note 8) | |
| - | | |
| - | |
| Stockholders’ Deficit: | |
| | | |
| | |
| Preferred stock, $.0001 par value, 2,400,000 authorized,
issued and outstanding | |
| 240 | | |
| 240 | |
| Common stock, $0.0001 par value, 10,000,000 authorized,
5,000,000 issued and outstanding | |
| 500 | | |
| 500 | |
| Additional paid-in capital | |
| 6,849,064 | | |
| 6,325,355 | |
| Accumulated deficit | |
| (11,388,956 | ) | |
| (9,147,967 | ) |
| Total stockholders’ deficit | |
| (4,539,152 | ) | |
| (2,821,872 | ) |
| Total Liabilities and Stockholders’ Deficit | |
$ | 1,254,868 | | |
$ | 605,298 | |
Scienture
Inc.
Statements
of Operations and Comprehensive Loss
| | |
| | |
| |
| | |
Year
Ended December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Net revenue | |
$ | 800,000 | | |
$ | 300,000 | |
| | |
| | | |
| | |
| Operating Expenses: | |
| | | |
| | |
| Research and development | |
| 2,029,812 | | |
| 3,061,493 | |
| General and administrative expenses | |
| 719,398 | | |
| 880,110 | |
| Total operating expenses | |
| 2,749,210 | | |
| 3,941,603 | |
| | |
| | | |
| | |
| Loss from Operations | |
| (1,949,210 | ) | |
| (3,641,603 | ) |
| | |
| | | |
| | |
| Other Income (Expense) | |
| | | |
| | |
| Dividend income | |
| 2,401 | | |
| - | |
| Interest income (expense), net | |
| (312,577 | ) | |
| (76,351 | ) |
| Miscellaneous income | |
| 18,397 | | |
| 9,574 | |
| Total other expense | |
| (291,779 | ) | |
| (66,777 | ) |
| | |
| | | |
| | |
| Net Loss | |
$ | (2,240,989 | ) | |
$ | (3,708,378 | ) |
| | |
| | | |
| | |
| Net loss per share - basic and diluted | |
| (0.45 | ) | |
| (0.74 | ) |
| Weighted-average shares used to compute net loss per share
- basic and diluted | |
| 5,000,000 | | |
| 5,000,000 | |
Scienture
Inc.
Statements
of Stockholders’ Deficit
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| | |
Preferred
Stock | | |
Common
Stock | | |
Additional
Paid-In | | |
Accumulated | | |
Total
Stockholders’ | |
| | |
Shares | | |
Amount | | |
Shares | | |
Amount | | |
Capital | | |
Deficit | | |
Deficit | |
| | |
| | |
| | |
| | |
| | |
| | |
| | |
| |
| Balance at December 31, 2021 | |
| 2,400,000 | | |
$ | 240 | | |
| 4,850,000 | | |
$ | 485 | | |
$ | 6,111,783 | | |
$ | (5,439,589 | ) | |
$ | 672,919 | |
| Common stock issued for services | |
| - | | |
| - | | |
| 150,000 | | |
| 15 | | |
| 145,485 | | |
| - | | |
| 145,500 | |
| Stock-based compensation expenses | |
| - | | |
| - | | |
| - | | |
| - | | |
| 68,087 | | |
| - | | |
| 68,087 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (3,708,378 | ) | |
| (3,708,378 | ) |
| Balance at December 31, 2022 | |
| 2,400,000 | | |
| 240 | | |
| 5,000,000 | | |
| 500 | | |
| 6,325,355 | | |
| (9,147,967 | ) | |
| (2,821,872 | ) |
| Balance | |
| 2,400,000 | | |
| 240 | | |
| 5,000,000 | | |
| 500 | | |
| 6,325,355 | | |
| (9,147,967 | ) | |
| (2,821,872 | ) |
| Warrants issued in connection with long-term convertible
debt | |
| - | | |
| - | | |
| - | | |
| - | | |
| 444,260 | | |
| - | | |
| 444,260 | |
| Stock-based compensation expenses | |
| - | | |
| - | | |
| - | | |
| - | | |
| 79,449 | | |
| - | | |
| 79,449 | |
| Net loss | |
| - | | |
| - | | |
| - | | |
| - | | |
| - | | |
| (2,240,989 | ) | |
| (2,240,989 | ) |
| Balance at December 31, 2023 | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000.00 | | |
$ | 500 | | |
$ | 6,849,064 | | |
$ | (11,388,956 | ) | |
$ | (4,539,152 | ) |
| Balance | |
| 2,400,000 | | |
$ | 240 | | |
| 5,000,000.00 | | |
$ | 500 | | |
$ | 6,849,064 | | |
$ | (11,388,956 | ) | |
$ | (4,539,152 | ) |
Scienture
Inc.
Statements
of Cash Flows
| | |
2023 | | |
2022 | |
| | |
Year
Ended December 31, | |
| | |
2023 | | |
2022 | |
| | |
| | |
| |
| Cash flows from operating activities: | |
| | | |
| | |
| Net loss | |
$ | (2,240,989 | ) | |
$ | (3,708,378 | ) |
| Adjustments to reconcile net loss to net cash used in operating
activities: | |
| | | |
| | |
| Common stock issued for services | |
| - | | |
| 145,500 | |
| Amortization of debt discount | |
| 69,378 | | |
| - | |
| Stock-based compensation expenses | |
| 79,449 | | |
| 68,087 | |
| Changes in operating assets and liabilities: | |
| | | |
| | |
| Accounts payable | |
| (286,501 | ) | |
| 65,394 | |
| Accrued expenses and other liabilities | |
| 248,716 | | |
| 76,704 | |
| Operating lease liability, net | |
| 206 | | |
| - | |
| Accounts receivable | |
| (66,414 | ) | |
| - | |
| Net cash used in operating activities | |
| (2,196,155 | ) | |
| (3,352,693 | ) |
| | |
| | | |
| | |
| Cash flows from financing activities: | |
| | | |
| | |
| Proceeds from the issuance of convertible notes | |
| 715,220 | | |
| 850,000 | |
| Proceeds from the issuance of long-term convertible debt | |
| 2,000,000 | | |
| - | |
| Net cash used in financing activities | |
| 2,715,220 | | |
| 850,000 | |
| | |
| | | |
| | |
| Net change in cash and cash equivalents | |
| 519,065 | | |
| (2,502,693 | ) |
| Cash and cash equivalents at beginning of year | |
| 604,813 | | |
| 3,107,506 | |
| Cash and cash equivalents at end of year | |
$ | 1,123,878 | | |
$ | 604,813 | |
| | |
| | | |
| | |
| Supplemental disclosure of cash flow information: | |
| | | |
| | |
| Cash paid for interest | |
$ | - | | |
$ | - | |
| Supplemental disclosure of non-cash financing activities: | |
| | | |
| | |
| Warrants issued in connection with long-term convertible
debt | |
$ | 444,260 | | |
$ | - | |
Note
1 Organization Overview and Basis of Presentation
Nature
of Operations
Scienture
Inc. (“the Company”) is a pharmaceutical research company which is engaged in the research and development of branded pharmaceutical
products. The IP application process of the company initiated in November 2019 and commenced the product development activities from
January 2020. The Company also plans to foray into commercialization of innovative and branded pharmaceutical products in the US market.
The
Company was incorporated in the state of Delaware in June 2019. The Company is headquartered in Hauppauge, New York, United States of
America.
Basis
of Presentation
The
Company’s fiscal year ends on December 31.
The
accompanying financial statements for the period ending December 31, 2023 and 2022 have been prepared in accordance with accounting principles
generally accepted in the United States (“U.S.GAAP”).
Note
2 Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S.GAAP requires the Company to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. These estimates are based on information available through the
date of the issuance of the financial statements and actual results could differ from those estimates. To the extent there are material
differences between the Company’s estimates and the actual results, the Company’s future consolidated results of operation
may be affected. Areas requiring significant estimates and assumptions by the Company include, but are not limited to:
| |
● |
fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| |
● |
accruals
for estimated liabilities; |
| |
● |
lease
term |
| |
● |
the
valuation of stock-based compensation awards ; and |
| |
● |
provisions
for income taxes and related valuation allowances and tax uncertainties. |
Liquidity
The
entity has just commenced operations and is expected to be funded by the stockholders for liquidity purposes. The liquidity position
of the entity is also dependent on the fundings by the additional development partners.
Comprehensive
Loss
Comprehensive
loss includes net loss as well as other changes in stockholder’s equity that result from transactions and economic events other
than those with stockholders. There was no difference between net loss and comprehensive loss presented in the financial statements for
the years ended December 31, 2023 and 2022.
Segment
Reporting
The
Company’s chief operating decision-maker is its Chief Executive Officer, who makes resource allocation decisions and assesses performance
based on financial information presented on an aggregate basis. There are no segment managers who are held accountable by the chief operating
decision-maker, or anyone else, for any planning, strategy and key decision-making regarding operations. Accordingly, the Company has
a single reportable segment and operating segment structure.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash
equivalents. Cash equivalents consist of amounts invested in money market funds and are stated at fair value.
Accounts
Receivable
Accounts
receivable consist of milestone payments due from development partners as a consideration for the rights granted for the commercialization
of the products to be developed. The Company reviews its accounts receivable and provides allowances of specific amounts if collectability
is no longer reasonably assured based on historical experience and specific collection issues. The allowance for doubtful accounts was
$0 as of December 31, 2023 and 2022, respectively.
(Refer
– Note 14– Subsequent Events – Termination of Exclusive License and Commercial Agreement).
Revenue
Recognition
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services.
The
Company adopted FASB ASU No. 2014-09, Revenue from Contracts with Customers and the related amendments, which are codified into ASC 606,
which establishes a broad principle that requires entities to assess the products or services promised in contracts with customers at
contract inception to determine the appropriate unit at which to record revenues, which is referred to as a performance obligation. Revenue
is recognized when control of the promised products or services is transferred to customers, at an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those products or services.
To
determine revenue recognition for arrangements that the Company determines are within the scope of ASC 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii)
determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it
is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the
customer. At contract inception, once the contract was determined to be within the scope of ASC 606, the Company assessed the goods or
services promised within each contract and determined those that were performance obligations, and assessed whether each promised good
or service was distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective
performance obligation when (or as) the performance obligation is satisfied.
A
performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in
ASC 606. The Company recognizes revenue at the point of sale of service.
Exclusive
License and Commercial Agreements
The
Company entered into an exclusive license and commercial agreement with Kesin Pharma Corporation, a related party where the Company granted
the exclusive license rights to commercialize SCN-102 in 2022 and SCN-104 in 2023 to Kesin (SCN-102 and SCN-104 are together referred
to as “the Products”) for use in the United States of America. In consideration of the rights granted, the Company is in
receipt of milestone payments and reimbursement of costs actually incurred related to the products. Revenue has been recognized when
such development milestone events take place and the amounts are due to be received. The Company recognized $800,000 and $300,000, respectively,
during the years ended December 31, 2023 and 2022 at the point when the development milestone events occurred. (Refer – Note 14–
Subsequent Events – Termination of Exclusive License and Commercial Agreement).
Fair
Value of Financial Instruments
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes
the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available.
Observable inputs are inputs that market participants would use in pricing the asset or liability and are developed based on market data
obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions of what
market participants would use in pricing the asset or liability based on the best information available in the circumstances. The financial
and nonfinancial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurement.
The hierarchy is presented down into three levels based on the reliability of the inputs.
| |
Level
1 |
Quoted
prices are available in active markets for identical assets or liabilities. |
| |
|
|
| |
Level
2 |
Observable
inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets
or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially
the full term of the assets or liabilities. |
| |
|
|
| |
Level
3 |
Unobservable
pricing inputs that are generally less observable from objective sources, such as discounted cash flow models or valuations. |
The
carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term convertible notes approximate their
fair value because of the short-term nature of these instruments. The carrying amount of long-term convertible debt approximate the fair
value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Concentration
of Credit Risks and Major Customers
Financial
instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The
Company places its cash and cash equivalents with financial institutions. During the years ended December 31, 2023, and 2022, the company
had one development partner that accounted for the entire revenue recognized in the Statement of Comprehensive Loss.
Research
& Development Expenses
Research
and development costs are expensed in the period incurred in accordance with ASC 730. Research and development expenses consist of independent
contractor costs , costs for outsourced analytical research and development activities, batch manufacturing cost and, advisory costs
as a part of research, market research costs and other regulatory consulting costs.
Stock-Based
Compensation
The
Company’s stock-based compensation expense relates to stock options. Stock-based compensation expense for its stock-based awards
is based on their grant date fair value. The fair values of stock-based compensations are recognized as compensation expense on a straight-line
basis over the requisite service period in which the awards are expected to vest. The Company estimates the fair value of stock option
awards on the grant date using the Black-Scholes option-pricing model. The Black-Scholes model considers several variables and assumptions
in estimating the fair value of stock-based awards. These variables include the per share fair value of the underlying common stock,
exercise price, expected term, risk-free interest rate, expected annual dividend yield and the expected stock price volatility over the
expected term. The Company has estimated volatility by reference to the historical volatilities of the Company and that of similar publicly
traded peer companies. The risk-free interest rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration
to the expected term of the equity-settled award.
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
The Company accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
Income
Taxes
State
Income Tax:
The
Company is incorporated in Delaware and headquartered in New York where the state tax is 8.70% and 7.25% respectively. However, due to
losses for the years ended December 31, 2023 and 2022, no provision on state income tax has been recognized.
Federal
Income Tax:
The
Company is a C Corporation for tax purposes, filing Form 1120 annually. Profits are not being passed through to owners. The company records
income taxes pursuant to the liability method. The Company has a loss before tax of ($2,240,989) and ($3,708,378) for years ended December
31, 2023 and 2022 respectively. Therefore, no provision for federal income tax has been recognized.
Deferred
Tax Assets and Liabilities
Deferred
tax assets and liabilities are determined based on the differences between the financial statement and the tax basis of assets and liabilities.
Realization of the future tax benefits related to the net deferred tax assets is dependent on many factors including the Company’s
ability to generate taxable income. Management believes that, at a minimum, it is more likely than not that future taxable income may
not be sufficient to realize the recorded assets.
The
Company has recorded a deferred tax asset related to its net operating loss carryforwards, timing difference between written down value
of assets, and unutilized R&D credit, which are expected to reduce future taxable income. The company has assessed the likelihood
of realizing the deferred tax assets and determined that it is more likely than not that a portion of the assets may not be realized.
Therefore, a valuation allowance has been created to account for 100% of the deferred tax assets to its expected realizable value.
The
impact of the deferred tax assets and related valuation allowance on the Company’s financial statements is as follows:
Schedule
of Deferred Tax Assets and Related Valuation Allowance
| | |
2023 | | |
2022 | |
| | |
Year
Ended December 31, | |
| | |
2023 | | |
2022 | |
| Deferred Tax Assets | |
| | | |
| | |
| Net operating loss carryforwards | |
$ | 3,173,840 | | |
$ | 2,491,667 | |
| Research and development tax credits | |
| 6,635 | | |
| 2,705 | |
| Property and equipment and operating lease liability | |
| 49,220 | | |
| 53,960 | |
| Valuation allowance | |
| (3,229,695 | ) | |
| (2,548,331 | ) |
| Net deferred tax assets | |
$ | - | | |
$ | - | |
Net
Loss per share
Basic
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period,
adjusted for outstanding shares that are subject to repurchase.
For
the calculation of diluted net loss per share, basic net loss per share is adjusted by the effect of dilutive securities if any. Diluted
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding and potential
common stock outstanding, if dilutive. For periods in which the Company reports net losses, diluted net loss per share is the same as
basic net loss per share because potentially dilutive shares of common stock are not assumed to have been issued if their effect is anti-dilutive.
Recent
Accounting Pronouncements
Accounting
Pronouncements Recently Adopted
The
Company has implemented all new relevant accounting pronouncements that are in effect through the date of these financial statements.
The pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not
believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial
position or results of operations.
In
June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (ASC 326), which provides guidance on measurement
of credit losses on financial instruments. This ASU adds a current expected credit loss impairment model to U.S.GAAP that is based on
expected losses rather than incurred losses whereby a broader range of reasonable and supportable information is required to be utilized
in order to derive credit loss estimates. The effective date of the new guidance as amended by ASU No. 2019-10 is fiscal years beginning
after December 15, 2022, including interim periods within those fiscal years. The Company adopted ASU 2016-13 effective January 1, 2023
the company determined that the update applied to trade receivables, but that there no material impact to the financial statements from
the adoption of ASU 2016-13.
In
February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No.
2016-2, Leases, to provide guidance for the accounting for leasing transactions. The standard requires the lessee to recognize a lease
liability along with a right-of-use asset for all leases with a term longer than one year. A lessee is permitted to make an accounting
policy election by class of underlying asset to not recognize the lease liability and related right-of-use asset for leases with a term
of one year or less. The provisions of this standard also apply to situations where the Company is the lessor. In March 2019, the FASB
issued ASU 2019-01, “Lease (842): Codification improvements.” This updated clarified that entities were exempt from disclosing
the effect of the change on income from continuing operations, net income, and related per-share amounts, if applicable, for interim
periods after the adoption of Accounting Standards Codification (“ASC”) 842.
The
standard was initially effective for annual and interim reporting periods beginning after December 15, 2019. However, in November 2019,
the FASB issued ASU 2019-10, “Financial Instruments Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases
(Topic 842): Effective Dates”, which deferred the effective date of ASU 2016-02 by an additional year. At its April 8, 2020, meeting,
the FASB voted to defer the effective date for ASC 842 another year. As such, the Company is required to adopt the new leases standard
for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022. The Company
adopted this new guidance effective January 1, 2022.
Accounting
Pronouncements Not Yet Adopted
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2023-07, “Segment
Reporting (Topic 280): Improvements to Reportable Segment Disclosures” (“ASU 2023-07”), which requires additional operating
segment disclosures in annual and interim consolidated financial statements. ASU 2023-07 is effective for annual periods beginning after
December 15, 2023 and for interim periods beginning after December 15, 2024 on a retrospective basis, with early adoption permitted.
The Company is evaluating the effect of adopting ASU 2023-07.
In
December 2023, the FASB issued Accounting Standards Update No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”
(“ASU 2023-09”), which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components
of the effective tax rate reconciliation and modifies other income tax-related disclosures. ASU 2023-09 is effective for annual periods
beginning after December 15, 2024 on a retrospective or prospective basis. The Company is evaluating the effect of adopting ASU 2023-09.
Note
3 Going Concern
The
Company has a net loss of ($2,240,989) for the year ended December 31,2023 and stockholders’ deficit of ($4,539,152) as of December
31, 2023. The Company’s situation raises a substantial doubt on whether the entity can continue as a going concern in the next
twelve months.
The
Company’s ability to continue as a going concern in the next twelve months following the date the financial statements were available
to be issued is dependent upon its ability to produce revenues and/or obtain financing sufficient to meet current and future obligations
and deploy such to produce profitable operating results.
Management
has evaluated these conditions and plans to generate revenues and raise capital as needed to satisfy its capital needs. During the next
twelve months, the Company intends to fund its operations through debt and/or equity financing.
There
are no assurances that management will be able to raise capital on terms acceptable to the Company. If it is unable to obtain sufficient
amount of additional capital, it may be required to reduce the scope of its planned development, which could harm its business, financial
condition, and operating results. The accompanying financial statements do not include any adjustments that might result from these uncertainties.
The
Company has evaluated whether there are certain conditions and events, considered in the aggregate, that raise substantial doubt about
the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
Note
4 Cash and Cash Equivalents
Cash
and cash equivalents consist of the following:
Schedule
of Cash and Cash Equivalents
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Balances with banks | |
$ | 31,943 | | |
$ | 604,813 | |
| Money market securities (Highly liquid investments) | |
| 1,091,935 | | |
| - | |
| Total Cash and Cash Equivalents | |
$ | 1,123,878 | | |
$ | 604,813 | |
Money
market securities were considered a Level 1 financial instrument.
Note
5 Convertible Notes
The
carrying value of the convertible notes approximate their fair value because of the short-term nature of these instruments. The convertible
notes issued bear an interest at a rate of 8% per annum and certain notes issued prior to 2022 bear an interest at a rate of 2% per annum.
As of December 31, 2023 and 2022, there were $3,665,220 and $2,950,000 in outstanding principal. All such notes have matured on December
31,2023. Interest expenses recognized for the years ended December 31, 2023 and 2022 amounted to $152,423 and $76,705 respectively. (Refer
– Note 14– Subsequent Events – Short-Term Convertible Notes)
Note
6 Long-Term Convertible Debt, net of debt discount
In
September 2023, the Company entered into a loan agreement with NVK Finance LLC, a Nebraska Limited Liability Company (‘NVK”)
for $2,000,000. The Board Member of the Company has significant influence in the decision making in NVK and hence considered as a related
party. The debt shall accrue interest at a per annum rate equal to Prime Rate plus 7 percent and the prime rates shall be adjusted quarterly
commencing on December 2023. As of December 31, 2023, the interest rate was 15.50%. The debt is collateralized by all of the Company’s
receivables, cash and cash equivalents and the title in Intellectual Property Rights and all proceeds thereof. The principal is entirely
repayable on the maturity date i.e. September 2025 and interest shall be paid monthly upon a Qualified Financing as defined in the Loan
Agreement. Interest expense related to the debt amounted to $95,583 for the year ended December 31, 2023 and the principal amount is
entirely outstanding as at December 31,2023. The outstanding balance under the NVK debt is convertible into common stock of the Company
at a fully-diluted Company valuation of $60,000,000.
In
connection with the NVK debt, the Company granted 509,014 warrants to purchase common stock. The fair value of the warrants was $444,260
using Black-Scholes option pricing model, which will be amortized to interest expense over the life of the notes. During the year ended
December 31, 2023, the Company amortized $69,377 of the debt discount to interest expense. (Refer – Note 11– Warrants)
Long-term
convertible debt, net of debt discount, consisted of the following:
Schedule of Long Term Convertible
Debt Net of Discount
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Principal | |
$ | 2,000,000 | | |
$ | - | |
| Less: Unamortized debt discount | |
| 374,883 | | |
| - | |
| Long-term convertible debt, net of debt discount | |
$ | 1,625,117 | | |
$ | - | |
Maturities
of the outstanding debt are as follows:
Schedule of Maturities Notes
| Years Ending December 31 | |
| |
| 2024 | |
$ | - | |
| Year 2 | |
$ | - | |
| 2025 | |
| 2,000,000 | |
| Year 3 | |
| 2,000,000 | |
| Total | |
$ | 2,000,000 | |
Note
7 Fair Value of Financial Instruments
The
carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and short-term convertible notes
approximate their fair value because of the short-term nature of these instruments. The carrying amount of long-term debt approximates
fair value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Note
8 Commitment and Contingencies
The
Company, in conjunction with its legal counsel, assesses the need to record a liability for litigation or loss contingencies. A liability
is recorded when and if it is determined that such a liability for litigation or loss contingencies is both probable and estimable. The
Company does not record any anticipated gains relating to its litigation or legal claims. The gains are only recorded upon receipt of
the settlement.
Although
the results of legal proceedings and claims cannot be predicted with certainty, the Company is not currently a party to any legal proceedings,
which would, individually or in the aggregate, have a material adverse effect on its results of operations, cash flows, or financial
position.
Note
9 Related Party Transactions
The
Company had entered into an Exclusive and Commercial agreement with Kesin Pharma Corporation (“Kesin”), in which one of the
company’s board member is the President and CEO, and had a significant influence in the decision making, which makes it a related
party. Sales made to Kesin as a part of milestone structure for the years ended December 31,2023 and 2022 amounted to $800,000 and $300,000
respectively.
The
Company has leased its office from Saptalis Pharmaceuticals LLC (“Saptalis”) , in which one of the Company’s Director
is the President and CEO. Lease payments made during the year ended December 31, 2023 and 2022 are $ 7200 and $0 respectively. The company
has also engaged Saptalis to provide development services and conduct testing and studies for the products under development by the Company.
Expenses incurred towards such testing and studies which is included in the Research and Development Expenses in the Statement of Comprehensive
Loss for the year ended December 31, 2023 and December 31,2022 amounted to $355,124 and $647,566 respectively.
Relatives
or related parties to directors purchased securities from the Company on the same terms as unrelated parties as set forth below:
Schedule of Relatives or Related
Parties to Directors Purchased Securities
| Name of the Related
Party | |
Nature of transaction | |
Transactions
during the year ended
December 31 | | |
Balances
as at
December 31 | |
| | |
| |
2023 | | |
2022 | | |
2022 | | |
2023 | |
| Ms. Pushpa Shankar | |
Issue of Preferred Stock | |
$ | - | | |
$ | - | | |
$ | 750,000 | | |
$ | 750,000 | |
| Ms. Pushpa Shankar | |
Issue of Convertible notes | |
| 400,000 | | |
| 150,000 | | |
| 550,000 | | |
| 400,000 | |
| Ms. Yogita Desai | |
Issue of Preferred Stock | |
| - | | |
| - | | |
| 500,000 | | |
| 500,000 | |
| Ms. Yogita Desai | |
Issue of Convertible notes | |
| - | | |
| - | | |
| 100,000 | | |
| 100,000 | |
| Mr. Sandeep Gupta | |
Issue of Convertible notes | |
| - | | |
| 50,000 | | |
| 50,000 | | |
| - | |
| Total | |
| |
$ | 400,000 | | |
$ | 200,000 | | |
$ | 1,950,000 | | |
$ | 1,750,000 | |
Note
10 Scienture Inc. 2020 Stock Option and Grant Plan
The
Stock Option and Grant Plan allows for the issuance of incentive stock options (“ISOs”), non-statutory stock options (“NSOs”).
ISOs may be granted only to the Company’s employees (including officers and directors who are also considered employees) and ex-employees.
NSOs may be granted to the Company’s employees and service providers such as advisors etc. Options under the Stock Option and Grant
Plan have a contractual term of not more than 10 years.
A
summary of the Company’s stock option activity under the Plans is as follows:
Summary of Stock Option Activity
| | |
Outstanding
Options | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Remaining
Term
(Years) | |
| Balance as of December 31, 2022 | |
| 560,000 | | |
$ | 0.83 | | |
| 7.95 | |
| Granted | |
| 185,000 | | |
| 1.13 | | |
| | |
| Exercised | |
| - | | |
| - | | |
| | |
| Cancelled and forfeited | |
| (90,000 | ) | |
| - | | |
| | |
| Balance as of December 31, 2023 | |
| 655,000 | | |
$ | 0.90 | | |
| 7.56 | |
| | |
| | | |
| | | |
| | |
| Vested and exercisable as of December 31, 2023 | |
| 410,065 | | |
$ | 0.84 | | |
| 6.84 | |
The
weighted-average grant date fair value of options granted during the years ended December 31, 2023 and 2022 are $ 0.55 and $ 0.52 per
share respectively.
The
Company recorded stock-based compensation expense in the Statement of Operations and Comprehensive Loss for the periods presented as
follows:
Schedule of Stock-based Compensation Expense
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| General and Administrative Expenses | |
$ | 79,449 | | |
$ | 68,087 | |
| Total stock-based compensation expense | |
$ | 79,449 | | |
$ | 68,087 | |
Stock
Option Valuation Assumptions
The
fair value of each employee option grant was estimated on the date of grant using the Black-Scholes option pricing model and the following
assumptions for the periods indicated:
Schedule of Stock Option Valuation Assumptions
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Expected volatility | |
| 65%
- 75 | % | |
| 65%
- 75 | % |
| Risk-free interest rate | |
| 0.5%
- 3.5 | % | |
| 0.5%
- 2.8 | % |
| Expected term | |
| 5.9
- 6.0 years | | |
| 5.9
- 6.0 years | |
| Expected dividend | |
| 0 | % | |
| 0 | % |
Note
11 Warrants
As
of December 31, 2023, there were 509,014 warrants outstanding and exercisable with an exercise price of $0.01 per share. The warrants
were granted in connection with the NVK debt (Refer - Note 6 - Long-Term Convertible Debt, net of debt discount).
The
following table summarizes the assumptions used to estimate the fair value of the outstanding warrants during the years ended December
31, 2023, and 2022:
Summary
of Assumptions Used to Estimate Fair Value of Warrants
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Expected dividend yield | |
| 0 | % | |
| - | |
| Weighted-average expected volatility | |
| 70.52 | % | |
| - | |
| Weighted-average risk-free interest rate | |
| 4.50 | % | |
| - | |
| Expected life of warrants | |
| 5
years | | |
| - | |
Note
12 Net Loss per Share
Stock
options to purchase 655,000 and 560,000 common stock, warrants to purchase 509,014 and 0 common stock, convertible preferred stock and
convertible notes to purchase 3,365,669 and 3,195,911 common stock and long-term convertible debt to purchase 9,529,683 and 0 common
stock were outstanding at December 31, 2023 and 2022, respectively, that were not included in the computation of diluted weighted average
common shares outstanding because their effect would have been anti-dilutive.
Note
13 Leases
The
Company determines if an arrangement is a lease at inception. Operating leases are included in Operating lease, Right of Use asset, Operating
Lease Liability (Current and Non-Current) in the Company’s balance sheets. The ROU assets represent the Company’s right to
use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising
from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments
over the lease term. As most of the leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate
at commencement date. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain
that the Company will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
As a practical expedient, the Company elected, for all office and facility leases, not to separate non-lease components from lease components
and instead to account for each separate lease component and its associated non-lease components as a single lease component. The Company
made an accounting policy election by class of underlying asset not to recognize the lease liability and related right-of-use asset for
leases with a term of one year or less.
The
Company has an operating lease for administrative office. The lease has remaining lease term around three years.
The
components of lease expense were as follows:
Schedule
of Lease Expense
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Operating lease costs | |
| | | |
| | |
| Amortization of ROU Assets | |
$ | 5,025 | | |
$ | - | |
| Interest on Lease Liabilities | |
$ | 2,380 | | |
$ | - | |
| Short term lease costs | |
$ | 34,021 | | |
$ | 32,677 | |
Supplemental
cash flow information related to leases was as follows:
Schedule
of Supplemental Cash Flow Information Related To Leases
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Cash paid for accounts included in the measurements of lease liabilities | |
| | |
| |
| Operating cash flows for Operating leases | |
$ | 7,200 | | |
$ | - | |
| Right of Use Assets obtained in exchange for new Lease
Liabilities | |
$ | 205 | | |
$ | - | |
Supplemental
balance sheet information related to leases was as follows:
Schedule
of Supplemental Balance Sheet Information Related To Leases
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Operating Leases | |
| | | |
| | |
| Right of Use Assets | |
$ | 64,091 | | |
$ | - | |
| | |
| | | |
| | |
| Short term Lease liabilities | |
$ | 21,404 | | |
$ | - | |
| Long term Lease liabilities | |
$ | 42,893 | | |
$ | - | |
| Total Lease Liabilities | |
$ | 64,297 | | |
$ | - | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term (in years) | |
| 2.83 | | |
| - | |
| | |
| | | |
| | |
| Weighted Average Discount Rate | |
| 15.50 | % | |
| - | |
Maturities
of lease liabilities were as follows at December 31, 2023:
Schedule
of Maturities Of Lease Liabilities
| December 31, 2023 | |
| |
| 2024 | |
$ | 29,017 | |
| 2025 | |
| 29,887 | |
| 2026 | |
| 17,823 | |
| Total lease payments | |
| 76,727 | |
| Less: Imputed interest | |
| 12,430 | |
| Total | |
$ | 64,297 | |
Note
14 Subsequent Events
Short-Term
Convertible Notes
All
the short-term convertible notes matured during the period of December 2023. The Company has not paid the amounts due including the principal
and the accrued interest. However, in March 2024, the Company had converted the outstanding principal of $3,665,220 and the accrued interest
till the date of conversion amounting to $276,233 into an aggregate of 965,568 preferred stock of the company.
Business
Combination
On
July 25, 2024, Scienture, Inc. (the “Company”) entered into and closed an Agreement and Plan of Merger (the “Merger
Agreement”) with MEDS, MEDS Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of MEDS (“Merger Sub I”)
and MEDS Merger Sub II, LLC, a Delaware limited liability company and wholly owned subsidiary of the Company (“Merger Sub II”).
Pursuant to the Merger Agreement, (i) Merger Sub I merged with and into the Company, with the Company continuing as the surviving entity
and a wholly owned subsidiary of MEDS, and (ii) the Company merged with and into Merger Sub II, with Merger Sub II continuing as the
surviving entity.
Termination
of Exclusive License and Commercial Agreement:
Scienture
Inc. (Scienture) and Kesin had entered into two exclusive license commercial agreements where Scienture had granted Kesin the rights
to commercialize the products. In March 2024, the parties have terminated the agreement, and the parties agreed that, Scienture shall
pay Kesin a total gross amount of $1,285,000 upon commercialization of product via a royalty arrangement.
This
agreement also requires that if the full $1,285,000 has not been repaid within two years of the early of i) commercial launch or ii)
120 days from FDA approval, then interest will accrue prospectively at a rate of 8% annually on unpaid balance. As of the date of issue
of financial statements, the entire amount is outstanding.
Management
has evaluated subsequent events through July 31, 2024, the date the financial statements were available to be issued.
Scienture
Holdings, Inc. (FKA TRxADE HEALTH, Inc.)
Unaudited
Pro Forma Financial Statements
The
following unaudited pro forma combined financial information presents the unaudited pro forma combined balance sheet and statement of
operations based upon the combined historical financial statements of the Scienture Holdings and Scienture LLC after giving effect to
the acquisition of Scienture LLC by Scienture Holdings (the “Transaction”) and the adjustments described in the accompanying
notes.
The
unaudited pro forma combined balance sheets of the Scienture Holdings and Scienture LLC as of June 30, 2024, have been prepared to reflect
the effects of the Transaction as if it occurred on June 30, 2024. The unaudited pro forma combined statements of operations for Scienture
Holdings and Scienture LLC for the six months ended June 30, 2024, combine the historical results and operations of the Scienture Holdings
and Scienture LLC giving effect to the Transaction as if it occurred on January 1, 2024. The unaudited pro forma combined statements
of operations for Scienture Holdings and Scienture LLC for the year ended December 31, 2023, combine the historical results and operations
of the Scienture Holdings and Scienture LLC giving effect to the Transaction as if it occurred on January 1, 2023.
The
unaudited pro forma combined financial information should be read in conjunction with the audited and unaudited historical financial
statements of Scienture Holdings and Scienture LLC and the notes thereto. Additional information about the basis of presentation of this
information is provided in Note 2 below.
The
unaudited pro forma combined financial information was prepared in accordance with Article 11 of Regulation S-X. The unaudited pro forma
adjustments reflecting the Transaction have been prepared in accordance with business combination accounting guidance as provided in
Accounting Standards Codification Topic 805, Business Combinations and reflect the preliminary allocation of the purchase price
to the acquired assets and liabilities based upon the preliminary estimate of fair values, using the assumptions set forth in the notes
to the unaudited pro forma combined financial information.
The
unaudited pro forma combined financial information is provided for informational purposes only and is not necessarily indicative of the
operating results or financial position that would have occurred if the transaction had been completed as of the dates set forth above,
nor is it indicative of the future results or financial position of the combined company. In connection with the pro forma financial
information, the Company allocated the purchase price using its best estimates of fair value. Accordingly, the pro forma acquisition
price adjustments are preliminary and subject to further adjustments as additional information becomes available and as additional analyses
are performed. The unaudited pro forma combined financial information also does not give effect to the potential impact of current financial
conditions, any anticipated synergies, operating efficiencies or cost savings that may result from the transaction or any integration
costs. Furthermore, the unaudited pro forma combined statements of operations do not include certain nonrecurring charges and the related
tax effects which result directly from the transaction as described in the notes to the unaudited pro forma combined financial information.
Unaudited
Proforma Combined Balance Sheet as of June 30, 2024
| | |
Scienture | | |
Scienture | | |
Pro Forma | | |
Pro Forma | |
| | |
Holdings | | |
LLC | | |
Adjustments | | |
Combined | |
| ASSETS | |
| | | |
| | | |
| | | |
| | |
| Current assets: | |
| | | |
| | | |
| | | |
| | |
| Cash | |
$ | 7,719,993 | | |
$ | 114,210 | | |
$ | - | | |
$ | 7,834,203 | |
| Accounts receivable, net | |
| 13,091 | | |
| - | | |
| - | | |
| 13,091 | |
| Inventory | |
| 6,439 | | |
| - | | |
| - | | |
| 6,439 | |
| Prepaid expenses | |
| 797,383 | | |
| - | | |
| - | | |
| 797,383 | |
| Notes receivable - related party | |
| 1,300,000 | | |
| - | | |
| - | | |
| 1,300,000 | |
| Other receivables | |
| 2,230,797 | | |
| 485 | | |
| - | | |
| 2,231,282 | |
| Deferred offering costs | |
| 69,444 | | |
| - | | |
| - | | |
| 69,444 | |
| Current assets of discontinued operations | |
| 7,297 | | |
| - | | |
| | | |
| 7,297 | |
| Total current assets | |
| 12,144,444 | | |
| 114,695 | | |
| - | | |
| 12,259,139 | |
| Property plant and equipment, net | |
| 6,500 | | |
| - | | |
| - | | |
| 6,500 | |
| Deposits | |
| 22,039 | | |
| - | | |
| - | | |
| 22,039 | |
| Deferred offering costs | |
| - | | |
| - | | |
| - | | |
| - | |
| Goodwill | |
| - | | |
| - | | |
| 7,234,860 | (a) | |
| 7,234,860 | |
| Intangible assets, net | |
| - | | |
| - | | |
| 76,400,000 | (a) | |
| 76,400,000 | |
| Investments | |
| 2,500,000 | | |
| - | | |
| - | | |
| 2,500,000 | |
| Operating lease right-of-use assets | |
| 175,550 | | |
| 61,579 | | |
| - | | |
| 237,129 | |
| Total assets | |
$ | 14,848,533 | | |
$ | 176,273 | | |
$ | 83,634,860 | | |
$ | 98,659,667 | |
| | |
| | | |
| | | |
| | | |
| | |
| LIABILITIES AND STOCKHOLDERS’ EQUITY (DEFICIT) | |
| | | |
| | | |
| | | |
| | |
| Current liabilities: | |
| | | |
| | | |
| | | |
| | |
| Accounts payable | |
$ | 726,266 | | |
$ | 884,581 | | |
$ | - | | |
$ | 1,610,847 | |
| Accrued liabilities | |
| 500,454 | | |
| 1,198,822 | | |
| - | | |
| 1,699,276 | |
| Other current liabilities | |
| 5,441 | | |
| - | | |
| - | | |
| 5,441 | |
| Lease liability - current | |
| 32,608 | | |
| 22,567 | | |
| - | | |
| 55,175 | |
| Warrant liability | |
| 1,631,974 | | |
| - | | |
| - | | |
| 1,631,974 | |
| Current liabilities of discontinued operations | |
| 5,346 | | |
| | | |
| | | |
| 5,346 | |
| Total current liabilities | |
| 2,902,089 | | |
| 2,105,970 | | |
| - | | |
| 5,008,059 | |
| Long-term convertible notes, net of debt discount | |
| - | | |
| 1,734,661 | | |
| - | | |
| 1,734,661 | |
| Lease liability | |
| 160,996 | | |
| 39,319 | | |
| - | | |
| 200,315 | |
| Development agreement liability | |
| - | | |
| 1,285,000 | | |
| - | | |
| 1,285,000 | |
| Total liabilities | |
| 3,063,085 | | |
| 5,164,950 | | |
| - | | |
| 8,228,035 | |
| | |
| | | |
| | | |
| | | |
| | |
| Stockholders’ equity (deficit): | |
| | | |
| | | |
| | | |
| | |
| | |
| | | |
| | | |
| 68 | (a) | |
| | |
| Preferred stock | |
| - | | |
| 337 | | |
| (337 | )(a) | |
| 68 | |
| Common stock | |
| 14 | | |
| 500 | | |
| 3 | (a) | |
| 17 | |
| | |
| | | |
| | | |
| (500 | )(a) | |
| - | |
| Additional paid-in capital | |
| 38,290,315 | | |
| 10,835,257 | | |
| 78,646,113 | (a) | |
| 116,936,428 | |
| | |
| | | |
| | | |
| (10,835,257 | )(a) | |
| - | |
| Accumulated deficit | |
| (26,504,881 | ) | |
| (15,824,770 | ) | |
| 15,824,770 | (a) | |
| (26,504,881 | ) |
| Total stockholders’ equity | |
| 11,785,448 | | |
| (4,988,676 | ) | |
| 83,634,860 | | |
| 90,431,632 | |
| Total liabilities and stockholders’ equity | |
$ | 14,848,533 | | |
$ | 176,273 | | |
$ | 83,634,860 | | |
$ | 98,659,667 | |
(a)
To record the purchase price allocation of the pro forma acquisition, including the recognition of goodwill and intangible assets, purchase
price consideration by the Scienture Holdings, and elimination of Scienture LLC’s equity.
Unaudited
Proforma Combined Statement of Operations for the Six Months Ended June 30, 2024
| | |
Scienture | | |
Scienture | | |
Pro Forma | | |
Pro Forma | |
| | |
Holdings | | |
LLC | | |
Adjustments | | |
Combined | |
| | |
| | |
| | |
| | |
| |
| Revenues | |
$ | 18,699 | | |
$ | - | | |
$ | - | | |
$ | 18,699 | |
| Cost of sales | |
| 19,402 | | |
| - | | |
| - | | |
| 19,402 | |
| Gross profit | |
| (703 | ) | |
| - | | |
| - | | |
| (703 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Wage and salary expense | |
| 534,644 | | |
| - | | |
| - | | |
| 534,644 | |
| Professional fees | |
| 688,689 | | |
| - | | |
| - | | |
| 688,689 | |
| Accounting and legal expense | |
| 510,755 | | |
| - | | |
| - | | |
| 510,755 | |
| Technology expense | |
| 138,289 | | |
| - | | |
| - | | |
| 138,289 | |
| Research and development | |
| - | | |
| 1,520,947 | | |
| - | | |
| 1,520,947 | |
| General and administrative | |
| 5,115,582 | | |
| 1,413,893 | | |
| - | | |
| 6,529,475 | |
| Termination fee | |
| - | | |
| 1,285,000 | | |
| - | | |
| 1,285,000 | |
| Total operating expenses | |
| 6,987,959 | | |
| 4,219,840 | | |
| - | | |
| 11,207,800 | |
| Operating loss | |
| (6,988,662 | ) | |
| (4,219,840 | ) | |
| - | | |
| (11,208,503 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | | |
| | | |
| | |
| Change in fair value of warrant liability | |
| (895,021 | ) | |
| - | | |
| - | | |
| (895,021 | ) |
| Interest and other income | |
| 103,952 | | |
| 11,931 | | |
| - | | |
| 115,883 | |
| Loss on disposal of asset | |
| (374,968 | ) | |
| - | | |
| - | | |
| (374,968 | ) |
| Interest expense | |
| (103,464 | ) | |
| (227,905 | ) | |
| - | | |
| (331,369 | ) |
| Total non-operating income (expense) | |
| (1,269,501 | ) | |
| (215,974 | ) | |
| - | | |
| (1,485,475 | ) |
| Net loss from continuing operations | |
| (8,258,163 | ) | |
| (4,435,814 | ) | |
| - | | |
| (12,693,977 | ) |
| Net income on discontinued operations | |
| 27,670,294 | | |
| - | | |
| - | | |
| 27,670,294 | |
| Net income/(loss) | |
$ | 19,412,131 | | |
$ | (4,435,814 | ) | |
| - | | |
$ | 14,976,317 | |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per common share from continuing operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (6.75 | ) | |
| | | |
| | | |
$ | (8.37 | ) |
| Diluted | |
$ | (6.75 | ) | |
| | | |
| | | |
$ | (8.37 | ) |
| Net income per common share from discontinued operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | 22.60 | | |
| | | |
| | | |
$ | 18.25 | |
| Diluted | |
$ | 19.02 | | |
| | | |
| | | |
$ | 15.85 | |
| Net income/(loss) | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | 15.86 | | |
| | | |
| | | |
$ | 9.88 | |
| Diluted | |
$ | 13.35 | | |
| | | |
| | | |
$ | 8.58 | |
| Weighted average common shares outstanding | |
| | | |
| | | |
| | | |
| | |
| Basic | |
| 1,224,337 | | |
| | | |
| | | |
| 1,515,892 | |
| Diluted | |
| 1,454,558 | | |
| | | |
| | | |
| 1,746,113 | |
Unaudited
Proforma Combined Statement of Operations for the Year Ended December 31, 2023
| | |
Scienture | | |
Scienture | | |
Pro Forma | | |
Pro Forma | |
| | |
Holdings | | |
LLC | | |
Adjustments | | |
Combined | |
| | |
| | |
| | |
| | |
| |
| Revenues | |
$ | 8,272,214 | | |
$ | 800,000 | | |
$ | - | | |
$ | 9,072,214 | |
| Cost of sales | |
| 5,673,957 | | |
| - | | |
| - | | |
| 5,673,957 | |
| Gross profit | |
| 2,598,257 | | |
| 800,000 | | |
| - | | |
| 3,398,257 | |
| | |
| | | |
| | | |
| | | |
| | |
| Operating expenses: | |
| | | |
| | | |
| | | |
| | |
| Wage and salary expense | |
| 2,698,178 | | |
| - | | |
| - | | |
| 2,698,178 | |
| Professional fees | |
| 1,466,567 | | |
| - | | |
| - | | |
| 1,466,567 | |
| Accounting and legal expense | |
| 1,534,377 | | |
| - | | |
| - | | |
| 1,534,377 | |
| Technology expense | |
| 1,376,908 | | |
| - | | |
| - | | |
| 1,376,908 | |
| Research and development | |
| - | | |
| 2,029,812 | | |
| - | | |
| 2,029,812 | |
| General and administrative | |
| 2,785,633 | | |
| 719,398 | | |
| - | | |
| 3,505,031 | |
| Total operating expenses | |
| 9,861,663 | | |
| 2,749,210 | | |
| - | | |
| 12,610,873 | |
| Operating loss | |
| (7,263,406 | ) | |
| (1,949,210 | ) | |
| - | | |
| (9,212,616 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Non-operating income (expense): | |
| | | |
| | | |
| | | |
| | |
| Change in fair value of warrant liability | |
| (148,420 | ) | |
| - | | |
| - | | |
| (148,420 | ) |
| Interest and other income | |
| 18,741 | | |
| 20,798 | | |
| - | | |
| 39,539 | |
| Goodwill impairment | |
| (5,129,115 | ) | |
| - | | |
| - | | |
| (5,129,115 | ) |
| Interest expense | |
| (1,198,346 | ) | |
| (312,577 | ) | |
| - | | |
| (1,510,923 | ) |
| Total non-operating income (expense) | |
| (6,457,140 | ) | |
| (291,779 | ) | |
| - | | |
| (6,748,919 | ) |
| Net loss from continuing operations | |
| (13,720,546 | ) | |
| (2,240,989 | ) | |
| - | | |
| (15,961,535 | ) |
| Net loss on discontinued operations | |
| (4,123,028 | ) | |
| - | | |
| - | | |
| (4,123,028 | ) |
| Net loss | |
$ | (17,843,574 | ) | |
$ | (2,240,989 | ) | |
$ | - | | |
$ | (20,084,563 | ) |
| | |
| | | |
| | | |
| | | |
| | |
| Net loss per common share from continuing operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (17.96 | ) | |
| | | |
| | | |
$ | (15.12 | ) |
| Diluted | |
$ | (5.76 | ) | |
| | | |
| | | |
$ | (5.97 | ) |
| Net loss per common share from discontinued operations | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (5.40 | ) | |
| | | |
| | | |
$ | (3.91 | ) |
| Diluted | |
$ | (1.73 | ) | |
| | | |
| | | |
$ | (1.54 | ) |
| Net loss | |
| | | |
| | | |
| | | |
| | |
| Basic | |
$ | (23.35 | ) | |
| | | |
| | | |
$ | (19.03 | ) |
| Diluted | |
$ | (7.49 | ) | |
| | | |
| | | |
$ | (7.51 | ) |
| Weighted average common shares outstanding | |
| | | |
| | | |
| | | |
| | |
| Basic | |
| 764,058 | | |
| | | |
| | | |
| 1,055,613 | |
| Diluted | |
| 2,381,443 | | |
| | | |
| | | |
| 2,672,998 | |
Note
1 – Description of Transaction and Basis of Presentation
Description
of Transaction
The
Transaction occurred pursuant to an Agreement and Plan of Merger (the “Merger Agreement”), which was entered into and closed
on July 25, 2024, by and among the Scienture Holdings, MEDS Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of
the Company (“Merger Sub I”), MEDS Merger Sub II, LLC, a Delaware limited liability company and wholly owned subsidiary of
the Company (“Merger Sub II”), and Scienture LLC. Pursuant to the Merger Agreement, on July 25, 2024, (i) Merger Sub I merged
with and into Scienture (the “First Merger”), with Scienture LLC continuing as the surviving entity and a wholly owned subsidiary
of the Company, and (ii) Scienture LLC merged with and into Merger Sub II (the “Second Merger” and, together with the First
Merger, the “Mergers”), with Merger Sub II continuing as the surviving entity and Merger Sub II changed its name to “Scienture,
LLC”.
As
consideration for the Transaction, at the effective time of the First Merger (the “First Effective Time”), the shares of
Scienture Holdings common stock issued and outstanding immediately prior to the First Effective Time were converted into the right
to receive, in the aggregate, (i) 291,536 shares of Scienture Holdings’ common stock, par value $0.00001 per share
representing 19.99% of the number of shares of Scienture Holdings’ common stock issued and outstanding immediately prior to
the First Effective Time, and (ii) 6,826,713 shares of the Series X Preferred Stock, each share of which is convertible into one
share of Scienture Holdings’ common stock.
Basis
of Presentation
The
historical financial information has been adjusted to give pro forma effect to events that are (i) directly attributable to the transaction,
(ii) factually supportable, and (iii) with respect to the unaudited proforma combined balance sheets and unaudited pro forma combined
statements of operations, expected to have a continuing impact on the combined results.
The
transaction was accounted for as a business acquisition wherein Scienture LLC is the accounting acquiree and Scienture Holdings is the
accounting acquirer.
Note
2 – Consideration Transferred
Scienture
Holdings issued 291,536 shares of its common stock and 6,826,713 shares of Series X Preferred Stock in connection with the Transaction.
The total fair value of the initial purchase price consideration associated with the Transaction was determined as follows:
| Common stock issued | |
$ | 3,221,245 | |
| Preferred stock issued | |
| 75,424,939 | |
| Total purchase price | |
$ | 78,646,184 | |
The
following table shows the preliminary allocation of the purchase price for Scienture LLC to the acquired net identifiable assets and
pro forma goodwill:
| Assets acquired | |
$ | 176,273 | |
| Goodwill | |
| 7,234,860 | |
| Intangible assets | |
| 76,400,000 | |
| Liabilities assumed | |
| (5,164,950 | ) |
| | |
$ | 78,646,184 | |
Scienture
Holdings recorded $7,234,860 in pro forma goodwill representing the remaining excess purchase price of the fair value of net assets acquired
and liabilities assumed. The pro forma intangible assets acquired consist of developed technology and the related intellectual property
and of the Company’s products. The Company is currently assessing whether the assets are indefinite-lived such as in-process research
and development assets, or whether they will begin amortization upon commercialization.
The purchase price allocation of intangible assets was evaluated under ASC 805, and was independently identified and valued by a third
party valuation expert. The identified intangible assets were determined to be product technologies, and were valued accordingly by each
product candidate:
| Product Candidate | |
Fair Value (in $000’s) | |
| SCN-102 | |
$ | 23,600 | |
| SCN-104 | |
| 25,000 | |
| SCN-106 | |
| 15,000 | |
| SCN-107 | |
| 12,800 | |
| SCN-106 | |
$ | 78,646,184 | |
The
fair value of the product technologies was determined by the Income Approach: Multi-Period Excess Earnings Methods (“MPEEM”).
The MPEEM measures economic benefits by calculating the cash flows attributable to an asset after deducting appropriate returns for contributory
assets used by the business in generating the asset’s revenue and earnings. The MPEEM utilized revenue and cash flow projections
through 2030 based on each product candidate’s phase of development.. Key assumptions include a 2% long-term revenue growth rate
and 3% contributory asset charge rate. The Company discounted the expected future cash flows at a 53.0% rate of return, equal to the
WACC plus 10%, to reflect the risk of the cash flows related to the product technologies. The Company then summed the present values
of the estimated future cash flows and included an amortization tax benefit to the value indication of each of the product technologies.
Prospectus

4,300,000 Shares
of Common Stock
January [●],
2025
PART II
INFORMATION
NOT REQUIRED IN PROSPECTUS
Item
13. Other Expenses of Issuance and Distribution.
The
following is an estimate of the expenses (all of which are to be paid by the registrant) that we may incur in connection with the securities
being registered hereby.
| | |
Amount Paid or to Be Paid | |
| SEC registration fee | |
$ | 4,845.31 | |
| Legal fees and expenses | |
$ | 25,000 | |
| Accounting fees and expenses | |
$ | 20,000 | |
| Printing fees and expenses | |
$ | 5,000 | |
| Total | |
$ | 54,845.31 | |
We
will bear all costs, expenses and fees in connection with the registration of the securities, including with regard to compliance with
state securities or “blue sky” laws. All amounts are estimates except the SEC registration fee.
Item
14. Indemnification of Directors and Officers.
Section
145 of the Delaware General Corporation Law (“DGCL”) authorizes a corporation’s Board of Directors to grant,
and authorizes a court to award, indemnity to officers, directors, and other corporate agents.
Pursuant
to the Company’s Certificate of Incorporation:
| |
● |
A
director of the Company shall, to the fullest extent permitted by the DGCL as it now exists or as it may hereafter be amended, not
be personally liable to the Company or its stockholders for monetary damages for breach of fiduciary duty as a director, except to
the extent such exception from liability is not permitted under the DGCL as the same exists or may hereafter be amended; and |
| |
|
|
| |
● |
To
the fullest extent permitted by applicable law, the Company is authorized to provide indemnification of, and advancement of expenses
to, such agents of the Company (and any other persons to which Delaware law permits the Company to provide indemnification) through
bylaw provisions, agreements with such agents or other persons, vote of stockholders or disinterested directors or otherwise, in
excess of the indemnification and advancement otherwise permitted by Section 145 of the DGCL, subject only to limits created by applicable
Delaware law (statutory or non-statutory), with respect to actions for breach of duty to the Company, its stockholders and others. |
Section
145 of the DGCL, provides, among other things, that a Delaware corporation may indemnify any person who was, is or is threatened to be
made, party to any threatened, pending or completed action, suit or proceeding, whether civil, criminal, administrative or investigative
(other than an action by or in the right of such corporation), by reason of the fact that such person is or was an officer, director,
employee or agent of such corporation or is or was serving at the request of such corporation as a director, officer, employee or agent
of another corporation or enterprise. The indemnity may include expenses (including attorneys’ fees), judgments, fines and amounts
paid in settlement actually and reasonably incurred by such person in connection with such action, suit or proceeding, provided such
person acted in good faith and in a manner he or she reasonably believed to be in or not opposed to the corporation’s best interests
and, with respect to any criminal action or proceeding, had no reasonable cause to believe that his or her conduct was unlawful. A Delaware
corporation may indemnify any persons who were or are a party to any threatened, pending or completed action or suit by or in the right
of the corporation by reason of the fact that such person is or was a director, officer, employee or agent of another corporation or
enterprise. The indemnity may include expenses (including attorneys’ fees) actually and reasonably incurred by such person in connection
with the defense or settlement of such action or suit, provided such person acted in good faith and in a manner he or she reasonably
believed to be in or not opposed to the corporation’s best interests, provided further that no indemnification is permitted without
judicial approval if the officer, director, employee or agent is adjudged to be liable to the corporation. Where an officer or director
is successful on the merits or otherwise in the defense of any action referred to above, the corporation must indemnify him or her against
the expenses (including attorneys’ fees) which such officer or director has actually and reasonably incurred.
Section
145 further authorizes a corporation to purchase and maintain insurance on behalf of any person who is or was a director, officer, employee
or agent of the corporation or is or was serving at the request of the corporation as a director, officer, employee or agent of another
corporation or enterprise, against any liability asserted against such person and incurred by such person in any such capacity, or arising
out of his or her status as such, whether or not the corporation would otherwise have the power to indemnify such person under Section
145.
The
indemnification rights set forth above are not exclusive of any other right which an indemnified person may have or hereafter acquire
under any statute, any provision of our amended and Certificate of Incorporation, our amended and restated Bylaws, agreement, vote of
stockholders or disinterested directors or otherwise. Notwithstanding the foregoing, we are not obligated to indemnify a director or
officer in respect of a proceeding (or part thereof) instituted by such director or officer, unless such proceeding (or part thereof)
has been authorized by the board of directors pursuant to the applicable procedure outlined in the amended and restated bylaws.
Section
174 of the DGCL provides, among other things, that a director, who willfully or negligently approves of an unlawful payment of dividends
or an unlawful stock purchase or redemption, may be held jointly and severally liable for such actions. A director who was either absent
when the unlawful actions were approved or dissented at the time may avoid liability by causing his or her dissent to such actions to
be entered in the books containing the minutes of the meetings of the Board of Directors at the time such action occurred or immediately
after such absent director receives notice of the unlawful acts.
The
Company’s policy is to enter into separate indemnification agreements with each of its directors and officers that provide the
maximum indemnity allowed to directors and executive officers by Section 145 of the DGCL and also to provide for certain additional procedural
protections. The Company also maintains directors’ and officers’ insurance to insure such persons against certain liabilities.
These indemnification provisions and the indemnification agreements may be sufficiently broad to permit indemnification of our officers
and directors for liabilities, including reimbursement of expenses incurred, arising under the Securities Act. However, insofar as indemnification
for liabilities arising under the Securities Act may be permitted to directors, officers and controlling persons of the Company pursuant
to the foregoing provisions, or otherwise, the Company has been advised that in the opinion of the Securities and Exchange Commission
such indemnification is against public policy as expressed in the Securities Act and is, therefore, unenforceable.
Item
15. Recent Sales of Unregistered Securities.
In
the three years preceding the filing of this registration statement, the Company has sold the following securities that were not registered
under the Securities Act and that were offered and sold pursuant to the exemptions from registration under the Securities Act:
2022
Warrant Exercises
In
January 2022, warrants to purchase 14,584 shares of common stock were exercised with an exercise price of $0.06 per share; the Company
issued 14,584 shares of common stock, and $875 in proceeds were received in connection with such exercise. Such issuances were made in
reliance on the exemptions from registration pursuant to Section 4(a)(2) of the Securities Act.
2022 SPA
On
October 4, 2022, the Company entered into a Securities Purchase Agreement (the “2022 SPA”) with an institutional investor.
The 2022 SPA provided for the sale and issuance by the Company of an aggregate of: (i) 920,000 shares of the Company’s Common Stock
(61,334 shares after the effect of the 1:15 reverse stock split on June 21, 2023), (ii) pre-funded warrants (the “Pre-Funded Warrants”)
to purchase up to 601,740 shares of common stock (40,116 shares after the effect of the 1:15 reverse stock split) (the “Shares”),
and (iii) private placement warrants (the “Private Placement Warrants”) to purchase up to 2,663,045 shares of common stock
(177,537 shares after the effect of the 1:15 reverse stock split). The offering price per Share was $1.15 and the offering price per
Pre-Funded Warrant was $1.14999. The Company received approximately $1.750 million in proceeds and paid approximately $0.205 million
in commissions and legal fees related to the transaction. The Private Placement Warrants were sold in a concurrent private placement.
In
January 2023, the investor executed the Pre-Funded Warrants and purchased 601,740 shares of common stock (40,116 shares after the effect
of the 1:15 reverse stock) with a purchase price of $6.02.
Such
issuances were made in reliance on the exemptions from registration pursuant to Section 4(a)(2) of the Securities Act.
Common
Stock Purchase Agreement
In
the first quarter of 2023, we issued 50,000 shares of common stock to White Lion Capital, LLC in connection with the termination of the
common stock purchase agreement dated September 7, 2022, as amended on September 12, 2022. Such issuances were made in reliance on the
exemptions from registration pursuant to Section 4(a)(2) of the Securities Act.
Superlatus
Merger Agreement
On
July 14, 2023, the Company entered into an Amended and Restated Agreement and Plan of Merger (the “Superlatus Merger Agreement”)
with Superlatus, Inc. (“Superlatus”) and Foods Merger Sub, Inc., a wholly-owned subsidiary of the Company (“Merger
Sub”). On July 31, 2023, the Company completed its acquisition of Superlatus in accordance with the terms and conditions of the
Merger Agreement (the “Merger”). Under the terms of the Merger Agreement, at the closing of the Merger (the “Closing”),
shareholders of Superlatus received in aggregate 136,441 shares of common stock of the Company and 306,855 shares of Company’s
Series B Preferred Stock, par value $0.00001 per share (the “Series B Preferred Stock”), with a conversion ratio of one to
one hundred. At Closing, the value of the common stock was $7.30 per share, resulting in a total value of $225,000,169.
Not
all of the closing conditions of the Merger Agreement were met. As a result, the Company entered into Amendment No. 1 to the Amended
and Restated Agreement and Plan of Merger (the “Amendment”) on January 8, 2024. Under the terms of the Amendment, the merger
consideration to the shareholders of Superlatus was adjusted to the aggregate of 136,441 shares of common stock of the Company and 15,759
shares of Company’s Series B Preferred Stock. At Closing, the value of the common stock was $7.30 per share, resulting in a total
value of $12,500,089. Additionally, the shareholders of Superlatus agreed to surrender back to the Company 291,096 shares of the Company’s
Series B Preferred Stock. In March 2024 the Company divested of its interest in Superlatus.
Such
issuances were made in reliance on the exemptions from registration pursuant to Section 4(a)(2) of the Securities Act.
2023
SPA
On
October 4, 2023, the Company entered into a Securities Purchase Agreement (the “2023 SPA”) with Hudson Global Ventures, LLC
(“Hudson”). Under the terms of the 2023 SPA, the Company agreed to sell, and Hudson agreed to purchase, 290 shares of Series
C Preferred Stock (the “Purchased Shares”) at a price of $1,000 per share and a warrant to purchase up to 41,193 shares of
common stock. Additionally, pursuant to the 2023 SPA, 40,000 shares of common stock were issued to Hudson upon closing for a commitment
fee. The Company received $250,000 in exchange for the Purchased Shares, common stock, and warrants, net of issuance costs.
On
July 12, 2024, the Company converted the 290 Purchased Shares into 52,158 shares of common stock at the election of the holder.
Such
issuances were made in reliance on the exemptions from registration pursuant to Section 4(a)(2) of the Securities Act.
Scienture
Merger Agreement
On
July 25, 2024, the Company, entered into and closed an Agreement and Plan of Merger (the “Scienture Merger Agreement”) with
MEDS Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of the Company (“Merger Sub I”), MEDS Merger
Sub II, LLC, a Delaware limited liability company and wholly owned subsidiary of the Company (“Merger Sub II” and, together
with Merger Sub I, the “Merger Subs”), and Scienture, Inc., a Delaware corporation (“Scienture”). Pursuant to
the Merger Agreement, (i) Merger Sub I merged with and into Scienture (the “First Merger”), with Scienture continuing as
the surviving entity and a wholly owned subsidiary of the Company, and (ii) Scienture merged with and into Merger Sub II (the “Second
Merger” and, together with the First Merger, the “Mergers”), with Merger Sub II continuing as the surviving entity.
As
consideration for the Mergers, at the effective time of the First Merger (the “First Effective Time”), the shares of Scienture
common stock issued and outstanding immediately prior to the First Effective Time were converted into the right to receive, in the aggregate,
(i) 291,536 shares of the Company’s common stock and (ii) 6,826,713 shares of the Company’s Series X Non-Voting Convertible
Preferred Stock, par value $0.00001 per share (the “Series X Preferred Stock”), each share of which was convertible into
one share of common stock.
On
September 20, 2024, all shares of Series X Preferred Stock were converted into a total of 6,826,753 shares of common stock.
Such
issuances were made in reliance on the exemptions from registration pursuant to Section 4(a)(2) of the Securities Act.
August
2024 Note and Warrants
In
August 2024, the Company issued a convertible note of $360,000, for which the Company received $314,000 in net proceeds. The Conversion
Price is the lesser of i) $8.36 or (ii) 85% of the lowest volume-weighted average prices of the preceding five trading days. The note
matures on August 20, 2025. In connection with the note, the Company issued 76,923 warrants to purchase common stock. The warrants have
an exercise price of $9.36 per share, are immediately exercisable and have a term of 5 years. Such issuances were made in reliance on
the exemptions from registration pursuant to Section 4(a)(2) of the Securities Act.
2024
Warrant Exercises
In
August 2024, the Company issued 28,571 shares of common stock pursuant to the exercise of warrants on a cashless basis. Such issuances
were made in reliance on the exemptions from registration pursuant to Section 4(a)(2) of the Securities Act.
Common
Stock Issued for Services
During
the nine months ended September 30, 2024, the Company issued 470,482 shares of common stock for services. The fair value of shares issued
for services were $4,450,919. Such issuances were made in reliance on the exemptions from registration pursuant to Section 4(a)(2) of
the Securities Act.
Item
16. Exhibits and Financial Statement Schedules.
(a) Exhibits.
| Exhibit
No. |
|
Description |
| 2.1 |
|
Agreement and Plan of Merger, dated July 25, 2024, by and among the Company, MEDS Merger Sub I, Inc., MEDS Merger Sub II, LLC, and Scienture, Inc. (incorporated by reference to Exhibit 2.1 of the Company’s Form 8-K filed on July 31, 2024). |
| |
|
|
| 3.1 |
|
Second
Amended and Restated Certificate of Incorporation of the Company, as amended through September 20, 2024 (incorporated by reference
to Exhibit 3.1 of the Company’s Form 10-Q filed on November 6, 2024). |
| |
|
|
| 3.2 |
|
Amended
and Restated Bylaws of the Company, as amended through March 24, 2022 (incorporated by reference to Exhibit 3.2 of the Company’s
Form 10-Q filed on November 6, 2024). |
| |
|
|
| 3.3 |
|
Certificate of Designation of Series B Preferred Stock (incorporated by reference to Exhibit 3.1 of the Company’s Form 8-K filed on June 26, 2023). |
| |
|
|
| 3.4 |
|
Certificate of Designation of Preference, Rights and Limitations of Series X Non-Voting Convertible Preferred Stock (incorporated by reference to Exhibit 3.1 of the Company’s Form 8-K filed on July 31, 2024). |
| |
|
|
| 5.1 |
|
Opinion of Dykema Gossett PLLC (incorporated by reference to Exhibit 5.1 of the Company’s Form S-1 filed on December 3, 2024). |
| |
|
|
| 10.1# |
|
Form of Indemnification Agreement entered into with certain directors and officers (incorporated by reference to Exhibit 10.1 of the Company’s Form 10-K filed on March 22, 2019). |
| |
|
|
| 10.2# |
|
Employment Agreement with Prashant Patel dated March 1, 2021 and effective March 31, 2024 (incorporated by reference to Exhibit 6.7 of the Company’s Form 1-A/A filed on November 6, 2024). |
| |
|
|
| 10.3# |
|
Employment Agreement with Surendra Ajjarapu dated April 14, 2020 (incorporated by reference to Exhibit 10.4 of the Company’s Form 8-K filed on April 16, 2020). |
| |
|
|
| 10.4# |
|
First Amendment to Employment Agreement with Surendra Ajjarapu dated May 5, 2020 and effective April 14, 2020 (incorporated by reference to Exhibit 10.2 of the Company’s Form 8-K filed on May 7, 2020). |
| |
|
|
| 10.5# |
|
Second Amendment to Employment Agreement with Mr. Ajjarapu dated August 29, 2022 and effective September 1, 2022 (incorporated by reference to Exhibit 10.3 of the Company’s Form 8-K filed on September 1, 2022). |
| |
|
|
| 10.6# |
|
Third Amendment to Employment Agreement with Surendra Ajjarapu dated January 17, 2023 and effective September 1, 2022 (incorporated by reference to Exhibit 10.4 of the Company’s Form 8-K filed on January 20, 2023). |
| |
|
|
| 10.7# |
|
Second Amended and Restated Trxade Group, Inc. 2019 Equity Incentive Plan, as amended through September 20, 2024 (incorporated by reference to Exhibit 10.7 of the Company’s Form S-1 filed on December 3, 2024). |
| |
|
|
| 10.8 |
|
Amended and Restated Agreement and Plan of Merger, dated July 14, 2023, between the Company, Foods Merger Sub, Inc., and Superlatus Inc. (incorporated by reference to Exhibit 2.1 of the Company’s Form 8-K filed on July 14, 2023). |
| |
|
|
| 10.9 |
|
Amendment No. 1 to the Amended and Restated Agreement and Plan of Merger by and between the Company, Superlatus Inc. and Foods Merger Sub Inc., dated January 8, 2024 (incorporated by reference to Exhibit 10.1 of the Company’s Form 8-K filed on January 11, 2024). |
| |
|
|
| 10.10 |
|
Asset Purchase Agreement between Trxade, Inc., Micro Merchant Systems, Inc. and the Company (for the limited purposes identified therein), dated February 16, 2024 (incorporated by reference to Exhibit 2.1 of the Company’s Form 8-K filed on February 16, 2024). |
| |
|
|
| 10.11 |
|
Subscription Agreement, dated February 29, 2024 between Trxade, Inc. and Lafayette Energy Corp. (incorporated by reference to Exhibit 10.1 of the Company’s Form 8-K filed on March 6, 2024). |
| 10.12 |
|
Stock Purchase Agreement, dated March 5, 2024 between the Company and Superlatus Foods Inc. (incorporated by reference to Exhibit 10.2 of the Company’s Form 8-K filed on March 6, 2024). |
| |
|
|
| 10.13 |
|
Form of Lock-Up Agreement (incorporated by reference to Exhibit 10.1 of the Company’s Form 8-K filed on July 31, 2024). |
| |
|
|
| 10.14# |
|
Consulting Agreement, dated July 25, 2024, by and between the Company and Surendra K. Ajjarapu (incorporated by reference to Exhibit 10.2 of the Company’s Form 8-K filed on July 31, 2024). |
| |
|
|
| 10.15# |
|
Consulting Agreement, dated July 25, 2024, by and between the Company and Prashant Patel (incorporated by reference to Exhibit 10.3 of the Company’s Form 8-K filed on July 31, 2024). |
| |
|
|
| 10.16 |
|
Form of Registration Rights Agreement (incorporated by reference to Exhibit 10.4 of the Company’s Form 8-K filed on July 31, 2024). |
| |
|
|
| 10.17 |
|
Assignment and Assumption of Membership Interests – Integra Pharma Solutions, LLC, dated October 4, 2024, by and between the Company and Softell Inc. (incorporated by reference to Exhibit 10.5 of the Company’s Form 10-Q filed on November 6, 2024). |
| |
|
|
| 10.18+ |
|
Purchase Agreement, dated November 25, 2024, between the Company and Arena Business Solutions Global SPC II, Ltd (incorporated by reference to Exhibit 10.6 of the Company’s Form 8-K filed on November 26, 2024). |
| |
|
|
| 10.19+ |
|
Securities Purchase Agreement, dated November 22, 2024, between the Company and the Arena Investors (incorporated by reference to Exhibit 10.1 of the Company’s Form 8-K filed on November 26, 2024). |
| |
|
|
| 10.20 |
|
Form of 10% Original Issue Discount Secured Convertible Debenture (incorporated by reference to Exhibit 10.2 of the Company’s Form 8-K filed on November 26, 2024). |
| |
|
|
| 10.21+ |
|
Security Agreement, dated November 25, 2024, between the Company and the Arena Investors (incorporated by reference to Exhibit 10.3 of the Company’s Form 8-K filed on November 26, 2024). |
| |
|
|
| 10.22 |
|
Guarantee Agreement, dated November 25, 2024, between Scienture, LLC and the Arena Investors (incorporated by reference to Exhibit 10.4 of the Company’s Form 8-K filed on November 26, 2024). |
| |
|
|
| 10.23 |
|
Registration Rights Agreement, dated November 25, 2024, between the Company and the Arena Investors (incorporated by reference to Exhibit 10.5 of the Company’s Form 8-K filed on November 26, 2024). |
| |
|
|
| 10.24 |
|
First Amendment of Loan and Security Agreement, dated November 22, 2024, between the Company, NVK Finance, LLC, Scienture, LLC, Srivatsav, LLC, and Shankar Hariharan (incorporated by reference to Exhibit 10.7 of the Company’s Form 8-K filed on November 26, 2024). |
| |
|
|
| 10.25*+ |
|
Master Services Agreement, dated October 29, 2024, by and between the Company and Anthem Biosciences Pvt. Ltd. |
| |
|
|
| 16.1 |
|
Letter from MaloneBailey, LLP to the Securities and Exchange Commission dated September 14, 2023 (incorporated by reference to Exhibit 16.1 of the Company’s Form 8-K, filed on September 14, 2023). |
| |
|
|
| 21.1 |
|
List of Subsidiaries (incorporated by reference to Exhibit 21.1 of the Company’s Form S-1 filed on December 3, 2024). |
| |
|
|
| 23.1* |
|
Consent of CM3 Advisory. |
| |
|
|
| 23.2* |
|
Consent of MaloneBailey, LLP. |
| |
|
|
| 23.3* |
|
Consent of Suri & Co. |
| |
|
|
| 23.4 |
|
Consent of Dykema Gossett PLLC (included as part of Exhibit 5.1). |
| |
|
|
| 24.1 |
|
Power of Attorney (reference is made to the signature page of the Registration Statement filed by the Company on December 3, 2024). |
| |
|
|
| 101.INS |
|
Inline
XBRL Instance Document - the instance document does not appear in the Interactive Data File because its XBRL tags are embedded within
the Inline XBRL document. |
| |
|
|
| 101.SCH |
|
Inline
XBRL Taxonomy Extension Schema Document |
| |
|
|
| 101.CAL |
|
Inline
XBRL Taxonomy Extension Calculation Linkbase Document |
| |
|
|
| 101.DEF |
|
Inline
XBRL Taxonomy Extension Definition Linkbase Document |
| |
|
|
| 101.LAB |
|
Inline
XBRL Taxonomy Extension Label Linkbase Document |
| |
|
|
| 101.PRE |
|
Inline
XBRL Taxonomy Extension Presentation Linkbase Document |
| |
|
|
| 104 |
|
Cover
Page Interactive Data File (embedded within the Inline XBRL document) |
| |
|
|
| 107 |
|
Filing Fee Table (incorporated by reference to Exhibit 107 of the Company’s Form S-1 filed on December 3, 2024). |
| * |
Filed
herewith. |
| |
|
| # |
Represents
management compensation plan, contract or arrangement. |
| |
|
| + |
Exhibits
and Schedules have been omitted pursuant to Item 601(a)(5) of Regulation S-K. The Company agrees to furnish supplementally a copy
of any omitted exhibit and schedule to the SEC upon request. |
Item
17. Undertakings.
(a)
The undersigned registrant hereby undertakes:
(1)
To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
(i)
To include any prospectus required by Section 10(a)(3) of the Securities Act of 1933;
(ii)
To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective
amendment thereof) which, individually or in the aggregate, represent a fundamental change in the information set forth in the registration
statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities
offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range
may be reflected in the form of prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate,
the changes in volume and price represent no more than 20% change in the maximum aggregate offering price set forth in the “Calculation
of Registration Fee” table in the effective registration statement; and
(iii)
To include any material information with respect to the plan of distribution not previously disclosed in the registration statement or
any material change to such information in the registration statement;
provided,
however, that paragraphs (a)(1)(i), (a)(1)(ii) and (a)(1)(iii) of this section do not apply if the information required to be
included in a post-effective amendment by those paragraphs is contained in reports filed with or furnished to the Securities and Exchange
Commission by the registrant pursuant to Section 13 or Section 15(d) of the Securities Exchange Act of 1934 that are incorporated by
reference in the registration statement, or is contained in a form of prospectus filed pursuant to Rule 424(b) that is part of the registration
statement;
(2)
That, for the purpose of determining any liability under the Securities Act of 1933, each such post-effective amendment shall be deemed
to be a new registration statement relating to the securities offered therein, and the offering of such securities at that time shall
be deemed to be the initial bona fide offering thereof;
(3)
To remove from registration by means of a post-effective amendment any of the securities being registered which remain unsold at the
termination of the offering;
(4)
That, for the purpose of determining liability under the Securities Act of 1933 to any purchaser:
(i)
Each prospectus filed by the registrant pursuant to Rule 424(b)(3) shall be deemed to be part of the registration statement as of the
date the filed prospectus was deemed part of and included in the registration statement; and
(ii)
Each prospectus required to be filed pursuant to Rule 424(b)(2), (b)(5), or (b)(7) as part of a registration statement in reliance on
Rule 430B relating to an offering made pursuant to Rule 415(a)(1)(i), (vii), or (x) for the purpose of providing the information required
by Section 10(a) of the Securities Act of 1933 shall be deemed to be part of and included in the registration statement as of the earlier
of the date such form of prospectus is first used after effectiveness or the date of the first contract of sale of securities in the
offering described in the prospectus. As provided in Rule 430B, for liability purposes of the issuer and any person that is at that date
an underwriter, such date shall be deemed to be a new effective date of the registration statement relating to the securities in the
registration statement to which that prospectus relates, and the offering of such securities at that time shall be deemed to be the initial
bona fide offering thereof; provided, however, that no statement made in a registration statement or prospectus that is
part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement
or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such effective
date, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement
or made in any such document immediately prior to such effective date;
(5)
That, for purposes of determining any liability under the Securities Act of 1933, each filing of the registrant’s annual report
pursuant to Section 13(a) or Section 15(d) of the Securities Exchange Act of 1934 (and, where applicable, each filing of an employee
benefit plan’s annual report pursuant to Section 15(d) of the Securities Exchange Act of 1934) that is incorporated by reference
in the registration statement shall be deemed to be a new registration statement relating to the securities offered therein, and the
offering of such securities at that time shall be deemed to be the initial bona fide offering thereof;
(6)
Insofar as indemnification for liabilities arising under the Securities Act of 1933 may be permitted to directors, officers and controlling
persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that in the opinion of
the Securities and Exchange Commission such indemnification is against public policy as expressed in the Securities Act of 1933 and is,
therefore, unenforceable. In the event that a claim for indemnification against such liabilities (other than the payment by the registrant
of expenses incurred or paid by a director, officer or controlling person of the registrant in the successful defense of any action,
suit or proceeding) is asserted by such director, officer or controlling person in connection with the securities being registered, the
registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate
jurisdiction the question whether such indemnification by it is against public policy as expressed in the Securities Act of 1933 and
will be governed by the final adjudication of such issue.
SIGNATURES
Pursuant
to the requirements of the Securities Act of 1933, as amended, the registrant has duly caused this registration statement to be signed
on its behalf by the undersigned, thereunto duly authorized, in the City of Tampa, Florida, on the 13th day of January
2025.
| |
SCIENTURE
HOLDINGS, INC. |
| |
|
|
| |
By: |
/s/
Surendra Ajjarapu |
| |
|
Surendra
Ajjarapu |
| |
|
Chief
Executive Officer |
Pursuant
to the requirements of the Securities Act of 1933, as amended, this registration statement has been signed by the following persons in
the capacities and on the dates indicated.
| Signature |
|
Title |
|
Date |
| |
|
|
|
|
| /s/
Surendra Ajjarapu |
|
Chief
Executive Officer |
|
January
13, 2025 |
| Surendra
Ajjarapu |
|
(Principal
Executive Officer) |
|
|
| |
|
|
|
|
| /s/
Prashant Patel |
|
Interim
Chief Financial Officer |
|
January
13, 2025 |
| Prashant
Patel |
|
(Principal
Financial and Accounting Officer) |
|
|
| |
|
|
|
|
* |
|
Director |
|
January
13, 2025 |
| Donald
G. Fell |
|
|
|
|
| |
|
|
|
|
* |
|
Director |
|
January
13, 2025 |
| Mayur
Doshi |
|
|
|
|
| |
|
|
|
|
* |
|
Director |
|
January
13, 2025 |
| Subbarao
Jayanthi |
|
|
|
|
| |
|
|
|
|
* |
|
Director |
|
January
13, 2025 |
| Shankar
Hariharan |
|
|
|
|
| |
|
|
|
|
* |
|
Director |
|
January
13, 2025 |
| Narasimhan
Mani |
|
|
|
|
Exhibit 10.25














Exhibit 23.1
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in this Registration Statement on Form S-1 of our report dated April 22, 2024 relating to
the financial statements of Scienture Holdings, Inc. (f/k/a TRxADE Health, Inc.), appearing in the Annual Report on Form 10-K of Scienture
Holdings, Inc. (f/k/a TRxADE Health, Inc.) for the year ended December 31, 2023. We also consent to the reference to us under the heading
“Experts” in such Registration Statement.
/s/
CM3 Advisory
San
Diego, California
January 13, 2025
Exhibit
23.2

CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We consent to the incorporation by reference in this Registration Statement on Form S-1 (Amendment No. 1) of our report dated March 27,
2023 with respect to the audited consolidated financial statements of Scienture Holdings, Inc. (f/k/a TRxADE HEALTH, Inc.) for the year
ended December 31, 2022. Our report contains an explanatory paragraph regarding the Company’s ability to continue as a going concern.
/s/
MaloneBailey, LLP
www.malonebailey.com
Houston,
Texas
January 13, 2025

Exhibit
23.3
CONSENT
OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
We hereby consent to the incorporation by reference in this Registration Statement on Form S-1 of Scienture Holdings, Inc. of our report
dated 31st July 2024 relating to the Financial Statements of Scienture, Inc. as of and for the years ended December 31, 2023 and 2022
which appears in this Registration Statement. We also consent to the reference to us under the heading “Experts” in such Registration
Statement.
/s/
Suri & Co., Chartered Accountants
No. 443 & 445 Guna Complex, Chennai
Date:
January 13, 2025
Place: Chennai, India
v3.24.4
Cover
|
6 Months Ended |
Jun. 30, 2024 |
|---|
| Entity Addresses [Line Items] |
|
| Document Type |
S-1/A
|
| Amendment Flag |
true
|
| Amendment Description |
Amendment No. 1
|
| Entity Registrant Name |
Scienture Holdings, Inc.
|
| Entity Central Index Key |
0001382574
|
| Entity Primary SIC Number |
5122
|
| Entity Tax Identification Number |
46-3673928
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
6308 Benjamin Rd
|
| Entity Address, Address Line Two |
Suite 708
|
| Entity Address, City or Town |
Tampa
|
| Entity Address, State or Province |
FL
|
| Entity Address, Postal Zip Code |
33634
|
| City Area Code |
(800)
|
| Local Phone Number |
261-0281
|
| Entity Filer Category |
Non-accelerated Filer
|
| Entity Small Business |
true
|
| Entity Emerging Growth Company |
false
|
| Business Contact [Member] |
|
| Entity Addresses [Line Items] |
|
| Entity Address, Address Line One |
6308 Benjamin Rd
|
| Entity Address, Address Line Two |
Suite 708
|
| Entity Address, City or Town |
Tampa
|
| Entity Address, State or Province |
FL
|
| Entity Address, Postal Zip Code |
33634
|
| City Area Code |
(800)
|
| Local Phone Number |
261-0281
|
| Contact Personnel Name |
Surendra Ajjarapu
|
| X |
- DefinitionDescription of changes contained within amended document.
| Name: |
dei_AmendmentDescription |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
dei_EntityAddressesLineItems |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate whether the registrant is one of the following: Large Accelerated Filer, Accelerated Filer, Non-accelerated Filer. Definitions of these categories are stated in Rule 12b-2 of the Exchange Act. This information should be based on the registrant's current or most recent filing containing the related disclosure. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityFilerCategory |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:filerCategoryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPrimary Standard Industrial Classification (SIC) Number for the Entity. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Form 40-F
-Number 249
-Section 240
-Subsection f
| Name: |
dei_EntityPrimarySicNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:sicNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicates that the company is a Smaller Reporting Company (SRC). Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntitySmallBusiness |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
v3.24.4
Balance Sheets - Scienture Inc [Member] - USD ($)
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Current Assets: |
|
|
|
| Cash and cash equivalents |
$ 114,210
|
$ 1,123,878
|
$ 604,813
|
| Accounts receivable |
|
66,414
|
|
| Other receivables |
485
|
485
|
485
|
| Total Current Assets |
114,695
|
1,190,777
|
605,298
|
| Operating lease, right of use asset |
61,579
|
64,091
|
|
| Total Assets |
176,274
|
1,254,868
|
605,298
|
| Current Liabilities: |
|
|
|
| Accounts payable |
884,581
|
107,175
|
393,676
|
| Accrued expenses and other liabilities |
1,198,822
|
332,211
|
83,494
|
| Convertible notes |
|
3,665,220
|
2,950,000
|
| Operating lease liability |
22,567
|
21,404
|
|
| Total Current Liabilities |
2,105,970
|
4,126,010
|
3,427,170
|
| Long-term convertible debt, net of debt discount |
1,734,661
|
1,625,117
|
|
| Operating lease liability, non current |
39,319
|
42,893
|
|
| Development agreement liability |
1,285,000
|
|
|
| Total Liabilities |
5,164,950
|
5,794,020
|
3,427,170
|
| Commitments and contingencies (Refer Note 8) |
|
|
|
| Stockholders’ Deficit: |
|
|
|
| Preferred stock, $.0001 par value, 2,400,000 authorized, issued and outstanding |
337
|
240
|
240
|
| Common stock, $0.0001 par value, 10,000,000 authorized, 5,000,000 issued and outstanding |
500
|
500
|
500
|
| Additional paid-in capital |
10,835,257
|
6,849,064
|
6,325,355
|
| Accumulated deficit |
(15,824,770)
|
(11,388,956)
|
(9,147,967)
|
| Total stockholders’ deficit |
(4,988,676)
|
(4,539,152)
|
(2,821,872)
|
| Total Liabilities and Stockholders’ Deficit |
$ 176,274
|
$ 1,254,868
|
$ 605,298
|
| X |
- DefinitionDevelopment agreement liability
| Name: |
SCNX_DevelopmentAgreementLiability |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Used to reflect the current portion of the liabilities (due within one year or within the normal operating cycle if longer). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
| Name: |
us-gaap_AccountsPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance for credit loss, of right to consideration from customer for product sold and service rendered in normal course of business, classified as current. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481990/310-10-45-2
| Name: |
us-gaap_AccountsReceivableNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expenses incurred but not yet paid nor invoiced, and liabilities classified as other.
| Name: |
us-gaap_AccruedLiabilitiesAndOtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of excess of issue price over par or stated value of stock and from other transaction involving stock or stockholder. Includes, but is not limited to, additional paid-in capital (APIC) for common and preferred stock. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_AdditionalPaidInCapital |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 12: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 13: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(12))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 30: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(11))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_Assets |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of asset recognized for present right to economic benefit, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 10: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_AssetsCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_AssetsCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionRepresents the caption on the face of the balance sheet to indicate that the entity has entered into (1) purchase or supply arrangements that will require expending a portion of its resources to meet the terms thereof, and (2) is exposed to potential losses or, less frequently, gains, arising from (a) possible claims against a company's resources due to future performance under contract terms, and (b) possible losses or likely gains from uncertainties that will ultimately be resolved when one or more future events that are deemed likely to occur do occur or fail to occur. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommitmentsAndContingencies |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable common stock (or common stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable common shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CommonStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amount of long-term convertible debt as of the balance sheet date, net of the amount due in the next twelve months or greater than the normal operating cycle, if longer. The debt is convertible into another form of financial instrument, typically the entity's common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ConvertibleDebtNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying value as of the balance sheet date of the portion of long-term debt due within one year or the operating cycle if longer identified as Convertible Notes Payable. Convertible Notes Payable is a written promise to pay a note which can be exchanged for a specified amount of another, related security, at the option of the issuer and the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_ConvertibleNotesPayableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liability recognized for present obligation requiring transfer or otherwise providing economic benefit to others. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(24))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(26))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 15: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(14))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 18: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 27: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 28: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 29: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
| Name: |
us-gaap_Liabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of liabilities and equity items, including the portion of equity attributable to noncontrolling interests, if any. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(32))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_LiabilitiesAndStockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionTotal obligations incurred as part of normal operations that are expected to be paid during the following twelve months or within one business cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481231/810-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 810
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481203/810-10-50-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 8: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-5
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 20: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
Reference 21: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481404/852-10-50-7
| Name: |
us-gaap_LiabilitiesCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_LiabilitiesCurrentAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after allowance, of receivables classified as other, due within one year or the operating cycle, if longer.
| Name: |
us-gaap_OtherReceivablesNetCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate par or stated value of issued nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer). This item includes treasury stock repurchased by the entity. Note: elements for number of nonredeemable preferred shares, par value and other disclosure concepts are in another section within stockholders' equity. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(21))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_PreferredStockValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of accumulated undistributed earnings (deficit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30)(a)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480016/944-40-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(23)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_RetainedEarningsAccumulatedDeficit |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- References
+ Details
| Name: |
us-gaap_StockholdersEquityAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Balance Sheets (Parenthetical) - Scienture Inc [Member] - $ / shares
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Preferred stock, par value |
$ 0.0001
|
$ 0.0001
|
$ 0.0001
|
| Preferred stock, shares authorized |
3,365,657
|
2,400,000
|
2,400,000
|
| Preferred stock, shares issued |
3,365,657
|
2,400,000
|
2,400,000
|
| Preferred stock, shares outstanding |
3,365,657
|
2,400,000
|
2,400,000
|
| Common stock, par value |
$ 0.0001
|
$ 0.0001
|
$ 0.0001
|
| Common stock, shares authorized |
10,000,000
|
10,000,000
|
10,000,000
|
| Common stock, shares issued |
5,000,000
|
5,000,000
|
5,000,000
|
| Common stock, shares outstanding |
5,000,000
|
5,000,000
|
5,000,000
|
| X |
- DefinitionFace amount or stated value per share of common stock. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of common shares permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_CommonStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionTotal number of common shares of an entity that have been sold or granted to shareholders (includes common shares that were issued, repurchased and remain in the treasury). These shares represent capital invested by the firm's shareholders and owners, and may be all or only a portion of the number of shares authorized. Shares issued include shares outstanding and shares held in the treasury. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_CommonStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares of common stock outstanding. Common stock represent the ownership interest in a corporation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_CommonStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionFace amount or stated value per share of preferred stock nonredeemable or redeemable solely at the option of the issuer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockParOrStatedValuePerShare |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe maximum number of nonredeemable preferred shares (or preferred stock redeemable solely at the option of the issuer) permitted to be issued by an entity's charter and bylaws. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
| Name: |
us-gaap_PreferredStockSharesAuthorized |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued for nonredeemable preferred shares and preferred shares redeemable solely at option of issuer. Includes, but is not limited to, preferred shares issued, repurchased, and held as treasury shares. Excludes preferred shares classified as debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 13
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-13
| Name: |
us-gaap_PreferredStockSharesIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAggregate share number for all nonredeemable preferred stock (or preferred stock redeemable solely at the option of the issuer) held by stockholders. Does not include preferred shares that have been repurchased. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(16)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
| Name: |
us-gaap_PreferredStockSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Statements of Operations And Comprehensive Loss - Scienture Inc [Member] - USD ($)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Net revenue |
|
$ 800,000
|
$ 800,000
|
$ 300,000
|
| Operating Expenses: |
|
|
|
|
| Research and development |
1,520,947
|
946,435
|
2,029,812
|
3,061,493
|
| General and administrative expenses |
1,413,893
|
267,236
|
719,398
|
880,110
|
| Termination fee |
1,285,000
|
|
|
|
| Total operating expenses |
4,219,840
|
1,213,671
|
2,749,210
|
3,941,603
|
| Loss from Operations |
(4,219,840)
|
(413,671)
|
(1,949,210)
|
(3,641,603)
|
| Other Income (Expense) |
|
|
|
|
| Dividend income |
|
|
2,401
|
|
| Other income |
11,931
|
18,304
|
|
|
| Interest income (expense), net |
(227,905)
|
(76,203)
|
(312,577)
|
(76,351)
|
| Miscellaneous income |
|
|
18,397
|
9,574
|
| Total other expense |
(215,974)
|
(57,899)
|
(291,779)
|
(66,777)
|
| Net Loss |
$ (4,435,814)
|
$ (471,570)
|
$ (2,240,989)
|
$ (3,708,378)
|
| Net loss per share - basic |
$ (0.89)
|
$ (0.09)
|
$ (0.45)
|
$ (0.74)
|
| Net loss per share - diluted |
$ (0.89)
|
$ (0.09)
|
$ (0.45)
|
$ (0.74)
|
| Weighted average shares used to compute net loss per share - basic |
5,000,000
|
5,000,000
|
5,000,000
|
5,000,000
|
| Weighted average shares used to compute net loss per share - diluted |
5,000,000
|
5,000,000
|
5,000,000
|
5,000,000
|
| X |
- DefinitionThe amount of net income (loss) for the period per each share of common stock or unit outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe amount of net income (loss) for the period available to each share of common stock or common unit outstanding during the reporting period and to each share or unit that would have been outstanding assuming the issuance of common shares or units for all dilutive potential common shares or units outstanding during the reporting period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 52
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-52
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 15
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482635/260-10-55-15
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (e)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-7
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(25))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(27))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(23))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-7
| Name: |
us-gaap_EarningsPerShareDiluted |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate total of expenses of managing and administering the affairs of an entity, including affiliates of the reporting entity, which are not directly or indirectly associated with the manufacture, sale or creation of a product or product line. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
| Name: |
us-gaap_GeneralAndAdministrativeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest income (expense) classified as nonoperating. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
| Name: |
us-gaap_InterestIncomeExpenseNonoperatingNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe aggregate amount of income or expense from ancillary business-related activities (that is to say, excluding major activities considered part of the normal operations of the business). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_NonoperatingIncomeExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionGenerally recurring costs associated with normal operations except for the portion of these expenses which can be clearly related to production and included in cost of sales or services. Includes selling, general and administrative expense.
| Name: |
us-gaap_OperatingExpenses |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OperatingExpensesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe net result for the period of deducting operating expenses from operating revenues. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 7: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 31
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-31
| Name: |
us-gaap_OperatingIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of income related to nonoperating activities, classified as other. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(7)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_OtherNonoperatingIncome |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_OtherNonoperatingIncomeExpenseAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe average number of shares or units issued and outstanding that are used in calculating diluted EPS or earnings per unit (EPU), determined based on the timing of issuance of shares or units in the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 16
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-16
| Name: |
us-gaap_WeightedAverageNumberOfDilutedSharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of [basic] shares or units, after adjustment for contingently issuable shares or units and other shares or units not deemed outstanding, determined by relating the portion of time within a reporting period that common shares or units have been outstanding to the total time in that period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-10
| Name: |
us-gaap_WeightedAverageNumberOfSharesOutstandingBasic |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Statements of Stockholders' Deficit - Scienture Inc [Member] - USD ($)
|
Preferred Stock [Member] |
Common Stock [Member] |
Additional Paid-in Capital [Member] |
Retained Earnings [Member] |
Stockholders Deficit [Member] |
| Balance at Dec. 31, 2021 |
$ 240
|
$ 485
|
$ 6,111,783
|
$ (5,439,589)
|
$ 672,919
|
| Balance, shares at Dec. 31, 2021 |
2,400,000
|
4,850,000
|
|
|
|
| Common stock issued for services |
|
$ 15
|
145,485
|
|
145,500
|
| Common stock issued for services, shares |
|
150,000
|
|
|
|
| Stock-based compensation expenses |
|
|
68,087
|
|
68,087
|
| Net loss |
|
|
|
(3,708,378)
|
(3,708,378)
|
| Balance, shares at Dec. 31, 2022 |
2,400,000
|
5,000,000
|
|
|
|
| Balance at Dec. 31, 2022 |
$ 240
|
$ 500
|
6,325,355
|
(9,147,967)
|
(2,821,872)
|
| Stock-based compensation expenses |
|
|
39,724
|
|
39,724
|
| Net loss |
|
|
|
(471,570)
|
(471,570)
|
| Balance at Jun. 30, 2023 |
240
|
500
|
6,365,079
|
(9,619,537)
|
(3,253,718)
|
| Balance at Dec. 31, 2022 |
$ 240
|
$ 500
|
6,325,355
|
(9,147,967)
|
(2,821,872)
|
| Balance, shares at Dec. 31, 2022 |
2,400,000
|
5,000,000
|
|
|
|
| Stock-based compensation expenses |
|
|
79,449
|
|
79,449
|
| Net loss |
|
|
|
(2,240,989)
|
(2,240,989)
|
| Warrants issued in connection with long-term convertible debt |
|
|
444,260
|
|
444,260
|
| Balance, shares at Dec. 31, 2023 |
2,400,000
|
5,000,000
|
|
|
|
| Balance at Dec. 31, 2023 |
$ 240
|
$ 500
|
6,849,064
|
(11,388,956)
|
(4,539,152)
|
| Stock-based compensation expenses |
|
|
44,837
|
|
44,837
|
| Net loss |
|
|
|
(4,435,814)
|
(4,435,814)
|
| Conversion of notes into preferred stock |
$ 97
|
|
3,941,356
|
|
3,941,453
|
| Conversion of notes into preferred stock, shares |
965,657
|
|
|
|
|
| Balance, shares at Jun. 30, 2024 |
3,365,657
|
5,000,000
|
|
|
|
| Balance at Jun. 30, 2024 |
$ 337
|
$ 500
|
$ 10,835,257
|
$ (15,824,770)
|
$ (4,988,676)
|
| X |
- DefinitionAmount of other increase (decrease) in additional paid in capital (APIC).
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalOther |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in additional paid in capital (APIC) resulting from the issuance of warrants. Includes allocation of proceeds of debt securities issued with detachable stock purchase warrants. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 470
-SubTopic 20
-Section 25
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481284/470-20-25-2
| Name: |
us-gaap_AdjustmentsToAdditionalPaidInCapitalWarrantIssued |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued which are neither cancelled nor held in the treasury.
| Name: |
us-gaap_SharesOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares issued during the period upon the conversion of units. An example of a convertible unit is an umbrella partnership real estate investment trust unit (UPREIT unit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfUnits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued in lieu of cash for services contributed to the entity. Number of shares includes, but is not limited to, shares issued for services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodSharesIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued during the period upon the conversion of units. An example of a convertible unit is an umbrella partnership real estate investment trust unit (UPREIT unit). Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueConversionOfUnits |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionValue of stock issued in lieu of cash for services contributed to the entity. Value of the stock issued includes, but is not limited to, services contributed by vendors and founders.
| Name: |
us-gaap_StockIssuedDuringPeriodValueIssuedForServices |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
v3.24.4
Statements of Cash Flows - Scienture Inc [Member] - USD ($)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Cash flows from operating activities: |
|
|
|
|
| Net loss |
$ (4,435,814)
|
$ (471,570)
|
$ (2,240,989)
|
$ (3,708,378)
|
| Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
| Common stock issued for services |
|
|
|
145,500
|
| Amortization of debt discount |
109,544
|
|
69,378
|
|
| Stock-based compensation expenses |
44,837
|
39,724
|
79,449
|
68,087
|
| Changes in operating assets and liabilities: |
|
|
|
|
| Accounts receivable |
66,414
|
(300,000)
|
(66,414)
|
|
| Accounts payable |
777,406
|
29,729
|
(286,501)
|
65,394
|
| Accrued expenses and other liabilities |
1,142,843
|
76,212
|
248,716
|
76,704
|
| Development agreement liability |
1,285,000
|
|
|
|
| Operating lease liability, net |
102
|
|
206
|
|
| Net cash used in operating activities |
(1,009,668)
|
(625,905)
|
(2,196,155)
|
(3,352,693)
|
| Cash flows from financing activities: |
|
|
|
|
| Proceeds from the issuance of convertible notes |
|
400,000
|
715,220
|
850,000
|
| Proceeds from the issuance of long-term convertible debt |
|
|
2,000,000
|
|
| Net cash used in financing activities |
|
400,000
|
2,715,220
|
850,000
|
| Net change in cash and cash equivalents |
(1,009,668)
|
(225,905)
|
519,065
|
(2,502,693)
|
| Cash and cash equivalents at beginning of year |
1,123,878
|
604,813
|
604,813
|
3,107,506
|
| Cash and cash equivalents at end of year |
114,210
|
378,908
|
1,123,878
|
604,813
|
| Supplemental disclosure of cash flow information: |
|
|
|
|
| Cash paid for interest |
|
|
|
|
| Supplemental disclosure of non-cash financing activities: |
|
|
|
|
| Conversion of notes and accrued interest into preferred stock |
$ 3,941,453
|
|
|
|
| Warrants issued in connection with long-term convertible debt |
|
|
$ 444,260
|
|
| X |
- DefinitionConversion of notes and accrued interest into preferred stock
| Name: |
SCNX_ConversionOfNotesAndAccruedInterestIntoPreferredStock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionIncrease decrease in development agreement liability.
| Name: |
SCNX_IncreaseDecreaseInDevelopmentAgreementLiability |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionWarrants issued in connection with long term convertible debt.
| Name: |
SCNX_WarrantsIssuedInConnectionWithLongtermConvertibleDebt |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_AdjustmentsToReconcileNetIncomeLossToCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense included in interest expense to amortize debt discount and premium associated with the related debt instruments. Excludes amortization of financing costs. Alternate captions include noncash interest expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(8))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1F
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1F
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-3
| Name: |
us-gaap_AmortizationOfDebtDiscountPremium |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash and cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including, but not limited to, disposal group and discontinued operations. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-8
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsIncludingDisposalGroupAndDiscontinuedOperations |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of increase (decrease) in cash, cash equivalents, and cash and cash equivalents restricted to withdrawal or usage; including effect from exchange rate change. Cash includes, but is not limited to, currency on hand, demand deposits with banks or financial institutions, and other accounts with general characteristics of demand deposits. Cash equivalents include, but are not limited to, short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-SubTopic 230
-Topic 830
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477401/830-230-45-1
| Name: |
us-gaap_CashCashEquivalentsRestrictedCashAndRestrictedCashEquivalentsPeriodIncreaseDecreaseIncludingExchangeRateEffect |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in the aggregate amount of liabilities incurred (and for which invoices have typically been received) and payable to vendors for goods and services received that are used in an entity's business. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsPayable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe increase (decrease) during the reporting period in amount due within one year (or one business cycle) from customers for the credit sale of goods and services. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccountsReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in accrued expenses, and obligations classified as other. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IncreaseDecreaseInAccruedLiabilitiesAndOtherOperatingLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_IncreaseDecreaseInOperatingCapitalAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase (decrease) in obligation for operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-SubTopic 20
-Topic 842
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_IncreaseDecreaseInOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash paid for interest, excluding capitalized interest, classified as operating activity. Includes, but is not limited to, payment to settle zero-coupon bond for accreted interest of debt discount and debt instrument with insignificant coupon interest rate in relation to effective interest rate of borrowing attributable to accreted interest of debt discount. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 17
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-17
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-2
| Name: |
us-gaap_InterestPaidNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionFair value of share-based compensation granted to nonemployees as payment for services rendered or acknowledged claims. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_IssuanceOfStockAndWarrantsForServicesOrClaims |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from financing activities, including discontinued operations. Financing activity cash flows include obtaining resources from owners and providing them with a return on, and a return of, their investment; borrowing money and repaying amounts borrowed, or settling the obligation; and obtaining and paying for other resources obtained from creditors on long-term credit. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInFinancingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash inflow (outflow) from operating activities, including discontinued operations. Operating activity cash flows include transactions, adjustments, and changes in value not defined as investing or financing activities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-24
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-25
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- References
+ Details
| Name: |
us-gaap_NetCashProvidedByUsedInOperatingActivitiesAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from the issuance of a long-term debt instrument which can be exchanged for a specified amount of another security, typically the entity's common stock, at the option of the issuer or the holder. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromConvertibleDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionThe cash inflow from a debt initially having maturity due after one year or beyond the operating cycle, if longer. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(i)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 14
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-14
| Name: |
us-gaap_ProceedsFromIssuanceOfLongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Organization Overview and Basis of Presentation
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Consolidation, Less than Wholly Owned Subsidiary, Parent Ownership Interest, Effects of Changes, Net [Line Items] |
|
|
| Organization Overview and Basis of Presentation |
Note
1 Organization Overview and Basis of Presentation
Nature
of Operations
Scienture
Inc. (“the Company”) is a pharmaceutical research company which is engaged in the research and development of branded pharmaceutical
products. The IP application process of the company initiated in November 2019 and commenced the product development activities from
January 2020. The Company also plans to foray into commercialization of innovative and branded pharmaceutical products in the US market.
The
Company was incorporated in the state of Delaware in June 2019. The Company is headquartered in Hauppauge, New York, United States of
America.
Basis
of Presentation
The
Company’s fiscal year ends on December 31.
The
accompanying financial statements for the periods ending June 30, 2024 and 2023 have been prepared in accordance with accounting principles
generally accepted in the United States (“U.S.GAAP”).
|
Note
1 Organization Overview and Basis of Presentation
Nature
of Operations
Scienture
Inc. (“the Company”) is a pharmaceutical research company which is engaged in the research and development of branded pharmaceutical
products. The IP application process of the company initiated in November 2019 and commenced the product development activities from
January 2020. The Company also plans to foray into commercialization of innovative and branded pharmaceutical products in the US market.
The
Company was incorporated in the state of Delaware in June 2019. The Company is headquartered in Hauppauge, New York, United States of
America.
Basis
of Presentation
The
Company’s fiscal year ends on December 31.
The
accompanying financial statements for the period ending December 31, 2023 and 2022 have been prepared in accordance with accounting principles
generally accepted in the United States (“U.S.GAAP”).
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_ConsolidationLessThanWhollyOwnedSubsidiaryParentOwnershipInterestEffectsOfChangesNetLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure for organization, consolidation and basis of presentation of financial statements disclosure. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480424/946-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480424/946-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 810
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/810/tableOfContent
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/205/tableOfContent
| Name: |
us-gaap_OrganizationConsolidationAndPresentationOfFinancialStatementsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Summary of Significant Accounting Policies
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Summary of Significant Accounting Policies |
Note
2 Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S.GAAP requires the Company to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. These estimates are based on information available through the
date of the issuance of the financial statements and actual results could differ from those estimates. To the extent there are material
differences between the Company’s estimates and the actual results, the Company’s future consolidated results of operation
may be affected. Areas requiring significant estimates and assumptions by the Company include, but are not limited to:
| |
● |
fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| |
● |
accruals
for estimated liabilities; |
| |
● |
the
valuation of stock-based compensation awards ; and |
| |
● |
provisions
for income taxes and related valuation allowances and tax uncertainties. |
Unaudited
Interim Financial Information
The
unaudited condensed consolidated interim financial statements and related notes have been prepared in accordance with U.S. GAAP for interim
financial information, within the rules and regulations of the United States Securities and Exchange Commission (the “SEC”).
Certain information and disclosures normally included in the annual financial statements prepared in accordance with U.S. GAAP have been
condensed or omitted pursuant to such rules and regulations. The unaudited interim financial statements have been prepared on a basis
consistent with the audited financial statements and in the opinion of management, reflect all adjustments, consisting of only normal
recurring adjustments, necessary for the fair presentation of the results for the interim periods presented and of the financial condition
as of the date of the interim balance sheet. The financial data and the other information disclosed in these notes to the interim financial
statements related to the six-month periods are unaudited. Unaudited interim results are not necessarily indicative of the results for
the full fiscal year.
The
accompanying unaudited interim condensed consolidated financial statements should be read in conjunction with the Company’s audited
financial statements and the notes thereto for the year ended December 31, 2023.
Liquidity
The
entity has just commenced operations and is expected to be funded by the stockholders for liquidity purposes. The liquidity position
of the entity is also dependent on the fundings by the additional development partners.
Comprehensive
Loss
Comprehensive
loss includes net loss as well as other changes in stockholder’s equity that result from transactions and economic events other
than those with stockholders. There was no difference between net loss and comprehensive loss presented in the financial statements for
the six months ended June 30, 2024 and 2023.
Segment
Reporting
The
Company’s chief operating decision-maker is its Chief Executive Officer, who makes resource allocation decisions and assesses performance
based on financial information presented on an aggregate basis. There are no segment managers who are held accountable by the chief operating
decision-maker, or anyone else, for any planning, strategy and key decision-making regarding operations. Accordingly, the Company has
a single reportable segment and operating segment structure.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash
equivalents. Cash equivalents consist of amounts invested in money market funds and are stated at fair value.
Accounts
Receivable
Accounts
receivable consist of milestone payments due from development partners as a consideration for the rights granted for the commercialization
of the products to be developed. The Company reviews its accounts receivable and provides allowances of specific amounts if collectability
is no longer reasonably assured based on historical experience and specific collection issues. The allowance for doubtful accounts was
$0 as of June 30, 2024 and December 31, 2023, respectively.
Revenue
Recognition
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services.
The
Company adopted FASB ASU No. 2014-09, Revenue from Contracts with Customers and the related amendments, which are codified into ASC 606,
which establishes a broad principle that requires entities to assess the products or services promised in contracts with customers at
contract inception to determine the appropriate unit at which to record revenues, which is referred to as a performance obligation. Revenue
is recognized when control of the promised products or services is transferred to customers, at an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those products or services.
To
determine revenue recognition for arrangements that the Company determines are within the scope of ASC 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii)
determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it
is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the
customer. At contract inception, once the contract was determined to be within the scope of ASC 606, the Company assessed the goods or
services promised within each contract and determined those that were performance obligations, and assessed whether each promised good
or service was distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective
performance obligation when (or as) the performance obligation is satisfied.
A
performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in
ASC 606. The Company recognizes revenue at the point of sale of service.
Exclusive
License and Commercial Agreements
The
Company entered into an exclusive license and commercial agreement with Kesin Pharma Corporation, a related party where the Company granted
the exclusive license rights to commercialize SCN-102 in 2022 and SCN-104 in 2023 to Kesin (SCN-102 and SCN-104 are together referred
to as “the Products”) for use in the United States of America. In consideration of the rights granted, the Company is in
receipt of milestone payments and reimbursement of costs actually incurred related to the products. Revenue has been recognized when
such development milestone events take place and the amounts are due to be received. The Company recognized $800,000 for the six months
ended June 30, 2023, at the point when the development milestone events occurred.
In
March 2024, the parties have terminated the agreement, and the parties agreed that, Scienture shall pay Kesin a total gross amount of
$1,285,000 upon commercialization of product via a royalty arrangement.
This
agreement also requires that if the full $1,285,00 has not been repaid within two years of the early of i) commercial launch or ii) 120
from FDA approval, then interest will accrue prospectively at a rate of 8% annually on unpaid balance. Accordingly, the Company recorded
a $1,285,000 termination fee liability. As of the date of issue of financial statements, the entire amount is outstanding.
Fair
Value of Financial Instruments
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes
the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available.
Observable inputs are inputs that market participants would use in pricing the asset or liability and are developed based on market data
obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions of what
market participants would use in pricing the asset or liability based on the best information available in the circumstances. The financial
and nonfinancial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurement.
The hierarchy is presented down into three levels based on the reliability of the inputs.
| |
Level
1 |
Quoted
prices are available in active markets for identical assets or liabilities. |
| |
|
|
| |
Level
2 |
Observable
inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets
or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially
the full term of the assets or liabilities. |
| |
|
|
| |
Level
3 |
Unobservable
pricing inputs that are generally less observable from objective sources, such as discounted cash flow models or valuations. |
The
carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term convertible notes approximate their
fair value because of the short-term nature of these instruments. The carrying amount of long-term convertible debt approximate the fair
value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Concentration
of Credit Risks and Major Customers
Financial
instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The
Company places its cash and cash equivalents with financial institutions. During the six months ended June 30, 2024, the Company had
one development partner that accounted for the entire revenue recognized in the Statement of Comprehensive Loss.
Research
& Development Expenses
Research
and development costs are expensed in the period incurred in accordance with ASC 730. Research and development expenses consist of independent
contractor costs , costs for outsourced analytical research and development activities, batch manufacturing cost and, advisory costs
as a part of research, market research costs and other regulatory consulting costs.
Stock-Based
Compensation
The
Company’s stock-based compensation expense relates to stock options. Stock-based compensation expense for its stock-based awards
is based on their grant date fair value. The Company estimates the fair value of stock option awards on the grant date using the Black-Scholes
option-pricing model. The Black-Scholes model considers several variables and assumptions in estimating the fair value of stock-based
awards. These variables include the per share fair value of the underlying common stock, exercise price, expected term, risk-free interest
rate, expected annual dividend yield and the expected stock price volatility over the expected term. The Company has estimated volatility
by reference to the historical volatilities of the Company and that of similar publicly traded peer companies. The risk-free interest
rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the equity-settled
award.
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
The Company accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
Net
Loss per share
Basic
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period,
adjusted for outstanding shares that are subject to repurchase.
For
the calculation of diluted net loss per share, basic net loss per share is adjusted by the effect of dilutive securities if any. Diluted
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding and potential
common stock outstanding, if dilutive. For periods in which the Company reports net losses, diluted net loss per share is the same as
basic net loss per share because potentially dilutive shares of common stock are not assumed to have been issued if their effect is anti-dilutive.
Disclosure - Schedule of Deferred Tax Assets and Related Valuation Allowance (Details)
Accounting
Pronouncements Not Yet Adopted
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2023-07, “Segment
Reporting (Topic 280): Improvements to Reportable Segment Disclosures” (“ASU 2023-07”), which requires additional operating
segment disclosures in annual and interim consolidated financial statements. ASU 2023-07 is effective for annual periods beginning after
December 15, 2023 and for interim periods beginning after December 15, 2024 on a retrospective basis, with early adoption permitted.
The Company is evaluating the effect of adopting ASU 2023-07.
In
December 2023, the FASB issued Accounting Standards Update No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”
(“ASU 2023-09”), which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components
of the effective tax rate reconciliation and modifies other income tax-related disclosures. ASU 2023-09 is effective for annual periods
beginning after December 15, 2024 on a retrospective or prospective basis. The Company is evaluating the effect of adopting ASU 2023-09.
|
Note
2 Summary of Significant Accounting Policies
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S.GAAP requires the Company to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. These estimates are based on information available through the
date of the issuance of the financial statements and actual results could differ from those estimates. To the extent there are material
differences between the Company’s estimates and the actual results, the Company’s future consolidated results of operation
may be affected. Areas requiring significant estimates and assumptions by the Company include, but are not limited to:
| |
● |
fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| |
● |
accruals
for estimated liabilities; |
| |
● |
lease
term |
| |
● |
the
valuation of stock-based compensation awards ; and |
| |
● |
provisions
for income taxes and related valuation allowances and tax uncertainties. |
Liquidity
The
entity has just commenced operations and is expected to be funded by the stockholders for liquidity purposes. The liquidity position
of the entity is also dependent on the fundings by the additional development partners.
Comprehensive
Loss
Comprehensive
loss includes net loss as well as other changes in stockholder’s equity that result from transactions and economic events other
than those with stockholders. There was no difference between net loss and comprehensive loss presented in the financial statements for
the years ended December 31, 2023 and 2022.
Segment
Reporting
The
Company’s chief operating decision-maker is its Chief Executive Officer, who makes resource allocation decisions and assesses performance
based on financial information presented on an aggregate basis. There are no segment managers who are held accountable by the chief operating
decision-maker, or anyone else, for any planning, strategy and key decision-making regarding operations. Accordingly, the Company has
a single reportable segment and operating segment structure.
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash
equivalents. Cash equivalents consist of amounts invested in money market funds and are stated at fair value.
Accounts
Receivable
Accounts
receivable consist of milestone payments due from development partners as a consideration for the rights granted for the commercialization
of the products to be developed. The Company reviews its accounts receivable and provides allowances of specific amounts if collectability
is no longer reasonably assured based on historical experience and specific collection issues. The allowance for doubtful accounts was
$0 as of December 31, 2023 and 2022, respectively.
(Refer
– Note 14– Subsequent Events – Termination of Exclusive License and Commercial Agreement).
Revenue
Recognition
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services.
The
Company adopted FASB ASU No. 2014-09, Revenue from Contracts with Customers and the related amendments, which are codified into ASC 606,
which establishes a broad principle that requires entities to assess the products or services promised in contracts with customers at
contract inception to determine the appropriate unit at which to record revenues, which is referred to as a performance obligation. Revenue
is recognized when control of the promised products or services is transferred to customers, at an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those products or services.
To
determine revenue recognition for arrangements that the Company determines are within the scope of ASC 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii)
determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it
is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the
customer. At contract inception, once the contract was determined to be within the scope of ASC 606, the Company assessed the goods or
services promised within each contract and determined those that were performance obligations, and assessed whether each promised good
or service was distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective
performance obligation when (or as) the performance obligation is satisfied.
A
performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in
ASC 606. The Company recognizes revenue at the point of sale of service.
Exclusive
License and Commercial Agreements
The
Company entered into an exclusive license and commercial agreement with Kesin Pharma Corporation, a related party where the Company granted
the exclusive license rights to commercialize SCN-102 in 2022 and SCN-104 in 2023 to Kesin (SCN-102 and SCN-104 are together referred
to as “the Products”) for use in the United States of America. In consideration of the rights granted, the Company is in
receipt of milestone payments and reimbursement of costs actually incurred related to the products. Revenue has been recognized when
such development milestone events take place and the amounts are due to be received. The Company recognized $800,000 and $300,000, respectively,
during the years ended December 31, 2023 and 2022 at the point when the development milestone events occurred. (Refer – Note 14–
Subsequent Events – Termination of Exclusive License and Commercial Agreement).
Fair
Value of Financial Instruments
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes
the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available.
Observable inputs are inputs that market participants would use in pricing the asset or liability and are developed based on market data
obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions of what
market participants would use in pricing the asset or liability based on the best information available in the circumstances. The financial
and nonfinancial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurement.
The hierarchy is presented down into three levels based on the reliability of the inputs.
| |
Level
1 |
Quoted
prices are available in active markets for identical assets or liabilities. |
| |
|
|
| |
Level
2 |
Observable
inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets
or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially
the full term of the assets or liabilities. |
| |
|
|
| |
Level
3 |
Unobservable
pricing inputs that are generally less observable from objective sources, such as discounted cash flow models or valuations. |
The
carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term convertible notes approximate their
fair value because of the short-term nature of these instruments. The carrying amount of long-term convertible debt approximate the fair
value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
Concentration
of Credit Risks and Major Customers
Financial
instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The
Company places its cash and cash equivalents with financial institutions. During the years ended December 31, 2023, and 2022, the company
had one development partner that accounted for the entire revenue recognized in the Statement of Comprehensive Loss.
Research
& Development Expenses
Research
and development costs are expensed in the period incurred in accordance with ASC 730. Research and development expenses consist of independent
contractor costs , costs for outsourced analytical research and development activities, batch manufacturing cost and, advisory costs
as a part of research, market research costs and other regulatory consulting costs.
Stock-Based
Compensation
The
Company’s stock-based compensation expense relates to stock options. Stock-based compensation expense for its stock-based awards
is based on their grant date fair value. The fair values of stock-based compensations are recognized as compensation expense on a straight-line
basis over the requisite service period in which the awards are expected to vest. The Company estimates the fair value of stock option
awards on the grant date using the Black-Scholes option-pricing model. The Black-Scholes model considers several variables and assumptions
in estimating the fair value of stock-based awards. These variables include the per share fair value of the underlying common stock,
exercise price, expected term, risk-free interest rate, expected annual dividend yield and the expected stock price volatility over the
expected term. The Company has estimated volatility by reference to the historical volatilities of the Company and that of similar publicly
traded peer companies. The risk-free interest rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration
to the expected term of the equity-settled award.
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
The Company accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
Income
Taxes
State
Income Tax:
The
Company is incorporated in Delaware and headquartered in New York where the state tax is 8.70% and 7.25% respectively. However, due to
losses for the years ended December 31, 2023 and 2022, no provision on state income tax has been recognized.
Federal
Income Tax:
The
Company is a C Corporation for tax purposes, filing Form 1120 annually. Profits are not being passed through to owners. The company records
income taxes pursuant to the liability method. The Company has a loss before tax of ($2,240,989) and ($3,708,378) for years ended December
31, 2023 and 2022 respectively. Therefore, no provision for federal income tax has been recognized.
Deferred
Tax Assets and Liabilities
Deferred
tax assets and liabilities are determined based on the differences between the financial statement and the tax basis of assets and liabilities.
Realization of the future tax benefits related to the net deferred tax assets is dependent on many factors including the Company’s
ability to generate taxable income. Management believes that, at a minimum, it is more likely than not that future taxable income may
not be sufficient to realize the recorded assets.
The
Company has recorded a deferred tax asset related to its net operating loss carryforwards, timing difference between written down value
of assets, and unutilized R&D credit, which are expected to reduce future taxable income. The company has assessed the likelihood
of realizing the deferred tax assets and determined that it is more likely than not that a portion of the assets may not be realized.
Therefore, a valuation allowance has been created to account for 100% of the deferred tax assets to its expected realizable value.
The
impact of the deferred tax assets and related valuation allowance on the Company’s financial statements is as follows:
Schedule
of Deferred Tax Assets and Related Valuation Allowance
| | |
2023 | | |
2022 | |
| | |
Year
Ended December 31, | |
| | |
2023 | | |
2022 | |
| Deferred Tax Assets | |
| | | |
| | |
| Net operating loss carryforwards | |
$ | 3,173,840 | | |
$ | 2,491,667 | |
| Research and development tax credits | |
| 6,635 | | |
| 2,705 | |
| Property and equipment and operating lease liability | |
| 49,220 | | |
| 53,960 | |
| Valuation allowance | |
| (3,229,695 | ) | |
| (2,548,331 | ) |
| Net deferred tax assets | |
$ | - | | |
$ | - | |
Net
Loss per share
Basic
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period,
adjusted for outstanding shares that are subject to repurchase.
For
the calculation of diluted net loss per share, basic net loss per share is adjusted by the effect of dilutive securities if any. Diluted
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding and potential
common stock outstanding, if dilutive. For periods in which the Company reports net losses, diluted net loss per share is the same as
basic net loss per share because potentially dilutive shares of common stock are not assumed to have been issued if their effect is anti-dilutive.
Recent
Accounting Pronouncements
Accounting
Pronouncements Recently Adopted
The
Company has implemented all new relevant accounting pronouncements that are in effect through the date of these financial statements.
The pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not
believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial
position or results of operations.
In
June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (ASC 326), which provides guidance on measurement
of credit losses on financial instruments. This ASU adds a current expected credit loss impairment model to U.S.GAAP that is based on
expected losses rather than incurred losses whereby a broader range of reasonable and supportable information is required to be utilized
in order to derive credit loss estimates. The effective date of the new guidance as amended by ASU No. 2019-10 is fiscal years beginning
after December 15, 2022, including interim periods within those fiscal years. The Company adopted ASU 2016-13 effective January 1, 2023
the company determined that the update applied to trade receivables, but that there no material impact to the financial statements from
the adoption of ASU 2016-13.
In
February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No.
2016-2, Leases, to provide guidance for the accounting for leasing transactions. The standard requires the lessee to recognize a lease
liability along with a right-of-use asset for all leases with a term longer than one year. A lessee is permitted to make an accounting
policy election by class of underlying asset to not recognize the lease liability and related right-of-use asset for leases with a term
of one year or less. The provisions of this standard also apply to situations where the Company is the lessor. In March 2019, the FASB
issued ASU 2019-01, “Lease (842): Codification improvements.” This updated clarified that entities were exempt from disclosing
the effect of the change on income from continuing operations, net income, and related per-share amounts, if applicable, for interim
periods after the adoption of Accounting Standards Codification (“ASC”) 842.
The
standard was initially effective for annual and interim reporting periods beginning after December 15, 2019. However, in November 2019,
the FASB issued ASU 2019-10, “Financial Instruments Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases
(Topic 842): Effective Dates”, which deferred the effective date of ASU 2016-02 by an additional year. At its April 8, 2020, meeting,
the FASB voted to defer the effective date for ASC 842 another year. As such, the Company is required to adopt the new leases standard
for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022. The Company
adopted this new guidance effective January 1, 2022.
Accounting
Pronouncements Not Yet Adopted
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2023-07, “Segment
Reporting (Topic 280): Improvements to Reportable Segment Disclosures” (“ASU 2023-07”), which requires additional operating
segment disclosures in annual and interim consolidated financial statements. ASU 2023-07 is effective for annual periods beginning after
December 15, 2023 and for interim periods beginning after December 15, 2024 on a retrospective basis, with early adoption permitted.
The Company is evaluating the effect of adopting ASU 2023-07.
In
December 2023, the FASB issued Accounting Standards Update No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”
(“ASU 2023-09”), which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components
of the effective tax rate reconciliation and modifies other income tax-related disclosures. ASU 2023-09 is effective for annual periods
beginning after December 15, 2024 on a retrospective or prospective basis. The Company is evaluating the effect of adopting ASU 2023-09.
|
| X |
- DefinitionThe entire disclosure for all significant accounting policies of the reporting entity. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 235
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/235/tableOfContent
| Name: |
us-gaap_SignificantAccountingPoliciesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Going Concern
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Consolidation, Less than Wholly Owned Subsidiary, Parent Ownership Interest, Effects of Changes, Net [Line Items] |
|
|
| Going Concern |
Note
3 Going Concern
The
Company has a net loss of ($4,435,814) for the six months ended June 30, 2024 and stockholders’ deficit of ($4,988,676) as of June
30, 2024. The Company’s situation raises a substantial doubt on whether the entity can continue as a going concern in the next
twelve months.
The
Company’s ability to continue as a going concern in the next twelve months following the date the financial statements were available
to be issued is dependent upon its ability to produce revenues and/or obtain financing sufficient to meet current and future obligations
and deploy such to produce profitable operating results.
Management
has evaluated these conditions and plans to generate revenues and raise capital as needed to satisfy its capital needs. During the next
twelve months, the Company intends to fund its operations through debt and/or equity financing.
There
are no assurances that management will be able to raise capital on terms acceptable to the Company. If it is unable to obtain sufficient
amount of additional capital, it may be required to reduce the scope of its planned development, which could harm its business, financial
condition, and operating results. The accompanying financial statements do not include any adjustments that might result from these uncertainties.
The
Company has evaluated whether there are certain conditions and events, considered in the aggregate, that raise substantial doubt about
the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
|
Note
3 Going Concern
The
Company has a net loss of ($2,240,989) for the year ended December 31,2023 and stockholders’ deficit of ($4,539,152) as of December
31, 2023. The Company’s situation raises a substantial doubt on whether the entity can continue as a going concern in the next
twelve months.
The
Company’s ability to continue as a going concern in the next twelve months following the date the financial statements were available
to be issued is dependent upon its ability to produce revenues and/or obtain financing sufficient to meet current and future obligations
and deploy such to produce profitable operating results.
Management
has evaluated these conditions and plans to generate revenues and raise capital as needed to satisfy its capital needs. During the next
twelve months, the Company intends to fund its operations through debt and/or equity financing.
There
are no assurances that management will be able to raise capital on terms acceptable to the Company. If it is unable to obtain sufficient
amount of additional capital, it may be required to reduce the scope of its planned development, which could harm its business, financial
condition, and operating results. The accompanying financial statements do not include any adjustments that might result from these uncertainties.
The
Company has evaluated whether there are certain conditions and events, considered in the aggregate, that raise substantial doubt about
the Company’s ability to continue as a going concern within one year after the date that the financial statements are issued.
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_ConsolidationLessThanWhollyOwnedSubsidiaryParentOwnershipInterestEffectsOfChangesNetLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe entire disclosure when substantial doubt is raised about the ability to continue as a going concern. Includes, but is not limited to, principal conditions or events that raised substantial doubt about the ability to continue as a going concern, management's evaluation of the significance of those conditions or events in relation to the ability to meet its obligations, and management's plans that alleviated or are intended to mitigate the conditions or events that raise substantial doubt about the ability to continue as a going concern. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 205
-SubTopic 40
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/205-40/tableOfContent
| Name: |
us-gaap_SubstantialDoubtAboutGoingConcernTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Cash and Cash Equivalents
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Cash and Cash Equivalents |
Note
4 Cash and Cash Equivalents
Cash
and cash equivalents consist of the following:
Schedule
of Cash and Cash Equivalents
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Balances with banks | |
$ | 116,107 | | |
$ | 31,943 | |
| Money market securities(Highly liquid investments) | |
| - | | |
| 1,091,935 | |
| Total Cash and Cash Equivalents | |
$ | 116,106 | | |
$ | 1,123,878 | |
Money
market securities were considered a Level 1 financial instrument.
|
Note
4 Cash and Cash Equivalents
Cash
and cash equivalents consist of the following:
Schedule
of Cash and Cash Equivalents
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Balances with banks | |
$ | 31,943 | | |
$ | 604,813 | |
| Money market securities (Highly liquid investments) | |
| 1,091,935 | | |
| - | |
| Total Cash and Cash Equivalents | |
$ | 1,123,878 | | |
$ | 604,813 | |
Money
market securities were considered a Level 1 financial instrument.
|
| X |
- DefinitionThe entire disclosure for cash and cash equivalent footnotes, which may include the types of deposits and money market instruments, applicable carrying amounts, restricted amounts and compensating balance arrangements. Cash and equivalents include: (1) currency on hand (2) demand deposits with banks or financial institutions (3) other kinds of accounts that have the general characteristics of demand deposits (4) short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Generally, only investments maturing within three months from the date of acquisition qualify. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_CashAndCashEquivalentsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Convertible Notes
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Convertible Notes |
Note
5 Convertible Notes
The
carrying value of the convertible notes approximate their fair value because of the short-term nature of these instruments. The convertible
notes issued bear an interest at a rate of 8% per annum and certain notes issued prior to 2022 bear an interest at a rate of 2% per annum.
As of December 31, 2023, there $3,665,220 in outstanding principal. All the short-term convertible notes matured during the period of
December 2023. In March 2024, the Company had converted the outstanding principal of $3,665,220 and the accrued interest through the
date of conversion amounting to $276,233 into an aggregate of 965,567 shares of preferred stock of the Company.
|
Note
5 Convertible Notes
The
carrying value of the convertible notes approximate their fair value because of the short-term nature of these instruments. The convertible
notes issued bear an interest at a rate of 8% per annum and certain notes issued prior to 2022 bear an interest at a rate of 2% per annum.
As of December 31, 2023 and 2022, there were $3,665,220 and $2,950,000 in outstanding principal. All such notes have matured on December
31,2023. Interest expenses recognized for the years ended December 31, 2023 and 2022 amounted to $152,423 and $76,705 respectively. (Refer
– Note 14– Subsequent Events – Short-Term Convertible Notes)
|
| X |
- DefinitionThe entire disclosure for information about short-term and long-term debt arrangements, which includes amounts of borrowings under each line of credit, note payable, commercial paper issue, bonds indenture, debenture issue, own-share lending arrangements and any other contractual agreement to repay funds, and about the underlying arrangements, rationale for a classification as long-term, including repayment terms, interest rates, collateral provided, restrictions on use of assets and activities, whether or not in compliance with debt covenants, and other matters important to users of the financial statements, such as the effects of refinancing and noncompliance with debt covenants. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-6
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 405
-SubTopic 40
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477092/405-40-50-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/470/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1C
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1C
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1I
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1I
| Name: |
us-gaap_DebtDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Long-Term Convertible Debt, net of debt discount
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Long-Term Convertible Debt, net of debt discount |
Note
6 Long-Term Convertible Debt, net of debt discount
In
September 2023, the Company entered into a loan agreement with NVK Finance LLC, a Nebraska Limited Liability Company (‘NVK”)
for $2,000,000. The Board Member of the Company has significant influence in the decision making in NVK and hence considered as a related
party. The debt shall accrue interest at a per annum rate equal to Prime Rate plus 7 percent and the prime rates shall be adjusted quarterly
commencing on December 2023. As of June 30, 2024 and December 31, 2023, the interest rate was 15.50%. The debt is collateralized by all
of the Company’s receivables, cash and cash equivalents and the title in Intellectual Property Rights and all proceeds thereof.
The principal is entirely repayable on the maturity date i.e. September 2025 and interest shall be paid monthly upon a Qualified Financing
as defined in the Loan Agreement. Interest expense related to the debt amounted to $95,583 for the year ended December 31, 2023 and the
principal amount is entirely outstanding as at December 31,2023. The outstanding balance under the NVK debt is convertible into common
stock of the Company at a fully-diluted Company valuation of $60,000,000.
In
connection with the NVK debt, the Company granted 509,014 warrants to purchase common stock. The fair value of the warrants was $444,260
using Black-Scholes option pricing model, which will be amortized to interest expense over the life of the notes. During the six months
ended June 30, 2024, the Company amortized $109,544 of the debt discount to interest expense.
Long-term
convertible debt, net of debt discount, consisted of the following:
Schedule of Long Term Convertible
Debt Net of Discount
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Principal | |
$ | 2,000,000 | | |
$ | 2,000,000 | |
| Less: Unamortized debt discount | |
| 265,339 | | |
| 374,883 | |
| Long-term convertible notes, net of debt discount | |
$ | 1,734,661 | | |
$ | 1,625,117 | |
Maturities
of the outstanding notes are as follows:
Schedule of Maturities Notes
| Years Ending December 31 | |
| |
| 2024 | |
$ | - | |
| Year 1 | |
$ | - | |
| 2025 | |
| 2,000,000 | |
| Year 3 | |
| | |
| Total | |
$ | 2,000,000 | |
|
Note
6 Long-Term Convertible Debt, net of debt discount
In
September 2023, the Company entered into a loan agreement with NVK Finance LLC, a Nebraska Limited Liability Company (‘NVK”)
for $2,000,000. The Board Member of the Company has significant influence in the decision making in NVK and hence considered as a related
party. The debt shall accrue interest at a per annum rate equal to Prime Rate plus 7 percent and the prime rates shall be adjusted quarterly
commencing on December 2023. As of December 31, 2023, the interest rate was 15.50%. The debt is collateralized by all of the Company’s
receivables, cash and cash equivalents and the title in Intellectual Property Rights and all proceeds thereof. The principal is entirely
repayable on the maturity date i.e. September 2025 and interest shall be paid monthly upon a Qualified Financing as defined in the Loan
Agreement. Interest expense related to the debt amounted to $95,583 for the year ended December 31, 2023 and the principal amount is
entirely outstanding as at December 31,2023. The outstanding balance under the NVK debt is convertible into common stock of the Company
at a fully-diluted Company valuation of $60,000,000.
In
connection with the NVK debt, the Company granted 509,014 warrants to purchase common stock. The fair value of the warrants was $444,260
using Black-Scholes option pricing model, which will be amortized to interest expense over the life of the notes. During the year ended
December 31, 2023, the Company amortized $69,377 of the debt discount to interest expense. (Refer – Note 11– Warrants)
Long-term
convertible debt, net of debt discount, consisted of the following:
Schedule of Long Term Convertible
Debt Net of Discount
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Principal | |
$ | 2,000,000 | | |
$ | - | |
| Less: Unamortized debt discount | |
| 374,883 | | |
| - | |
| Long-term convertible debt, net of debt discount | |
$ | 1,625,117 | | |
$ | - | |
Maturities
of the outstanding debt are as follows:
Schedule of Maturities Notes
| Years Ending December 31 | |
| |
| 2024 | |
$ | - | |
| Year 2 | |
$ | - | |
| 2025 | |
| 2,000,000 | |
| Year 3 | |
| 2,000,000 | |
| Total | |
$ | 2,000,000 | |
|
| X |
- DefinitionThe entire disclosure for long-term debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 470
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/470/tableOfContent
| Name: |
us-gaap_LongTermDebtTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Fair Value of Financial Instruments
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Fair Value of Financial Instruments |
Note
7 Fair Value of Financial Instruments
The
carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and short-term convertible notes
approximate their fair value because of the short-term nature of these instruments. The carrying amount of long-term debt approximates
fair value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
|
Note
7 Fair Value of Financial Instruments
The
carrying amounts of cash and cash equivalents, accounts receivable, accounts payable, accrued expenses and short-term convertible notes
approximate their fair value because of the short-term nature of these instruments. The carrying amount of long-term debt approximates
fair value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
|
| X |
- DefinitionThe entire disclosure for the fair value of financial instruments (as defined), including financial assets and financial liabilities (collectively, as defined), and the measurements of those instruments as well as disclosures related to the fair value of non-financial assets and liabilities. Such disclosures about the financial instruments, assets, and liabilities would include: (1) the fair value of the required items together with their carrying amounts (as appropriate); (2) for items for which it is not practicable to estimate fair value, disclosure would include: (a) information pertinent to estimating fair value (including, carrying amount, effective interest rate, and maturity, and (b) the reasons why it is not practicable to estimate fair value; (3) significant concentrations of credit risk including: (a) information about the activity, region, or economic characteristics identifying a concentration, (b) the maximum amount of loss the entity is exposed to based on the gross fair value of the related item, (c) policy for requiring collateral or other security and information as to accessing such collateral or security, and (d) the nature and brief description of such collateral or security; (4) quantitative information about market risks and how such risks are managed; (5) for items measured on both a recurring and nonrecurring basis information regarding the inputs used to develop the fair value measurement; and (6) for items presented in the financial statement for which fair value measurement is elected: (a) information necessary to understand the reasons for the election, (b) discussion of the effect of fair value changes on earnings, (c) a description of [similar groups] items for which the election is made and the relation thereof to the balance sheet, the aggregate carrying value of items included in the balance sheet that are not eligible for the election; (7) all other required (as defined) and desired information. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 107
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-107
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 100
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-100
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2E
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6A
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-6A
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 940
-SubTopic 820
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478119/940-820-50-1
| Name: |
us-gaap_FairValueDisclosuresTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Commitment and Contingencies
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Commitment and Contingencies |
Note
8 Commitment and Contingencies
The
Company, in conjunction with its legal counsel, assesses the need to record a liability for litigation or loss contingencies. A liability
is recorded when and if it is determined that such a liability for litigation or loss contingencies is both probable and estimable. The
Company does not record any anticipated gains relating to its litigation or legal claims. The gains are only recorded upon receipt of
the settlement.
Although
the results of legal proceedings and claims cannot be predicted with certainty, the Company is not currently a party to any legal proceedings,
which would, individually or in the aggregate, have a material adverse effect on its results of operations, cash flows, or financial
position.
In
August 2024, Kesin demanded immediate payment of the full amount under the Kesin Termination Agreement, alleging the full amount is payable
in connection with the consummation Scienture’s business combination with the Company. Scienture has disputed that the amount is
now payable, and the parties are in discussions to resolve the issue. There can be no assurance that an amicable resolution will be obtained.
If Kesin brings a legal action, Scienture will vigorously defend it.
|
Note
8 Commitment and Contingencies
The
Company, in conjunction with its legal counsel, assesses the need to record a liability for litigation or loss contingencies. A liability
is recorded when and if it is determined that such a liability for litigation or loss contingencies is both probable and estimable. The
Company does not record any anticipated gains relating to its litigation or legal claims. The gains are only recorded upon receipt of
the settlement.
Although
the results of legal proceedings and claims cannot be predicted with certainty, the Company is not currently a party to any legal proceedings,
which would, individually or in the aggregate, have a material adverse effect on its results of operations, cash flows, or financial
position.
|
| X |
- DefinitionThe entire disclosure for commitments and contingencies. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 405
-SubTopic 30
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/405-30/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 450
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/450/tableOfContent
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 954
-SubTopic 440
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478522/954-440-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 440
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482648/440-10-50-4
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 440
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/440/tableOfContent
| Name: |
us-gaap_CommitmentsAndContingenciesDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Related Party Transactions
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Related Party Transactions |
Note
9 Related Party Transactions
The
Company had entered into an Exclusive and Commercial agreement with Kesin Pharma Corporation (“Kesin”), in which one of the
company’s board member is the President and CEO, and had a significant influence in the decision making, which makes it a related
party. Sales made to Kesin as a part of milestone structure for the six months ended June 30, 2024 and 2023 were $0 and $800,000, respectively.
The Company terminated the agreement with Kesin in March 2024, and recorded a termination fee and related liability of $1,285,000 as
of June 30, 2024.
The
Company has leased its office from Saptalis Pharmaceuticals LLC (“Saptalis”) , in which one of the Company’s Director
is the President and CEO. Lease payments made during the six months ended June 30, 2024 and 2023 are $14,440 and $0, respectively. The
Company has also engaged Saptalis to provide development services and conduct testing and studies for the products under development
by the Company. Expenses incurred towards such testing and studies which is included in the Research and Development Expenses in the
Statement of Comprehensive Loss for the six months ended June 30, 2024 and 2023 amounted to $106,539 and $189,027, respectively.
During
the six months ended June 30, 2023, a related party to a director issued a convertible note to the Company for $250,000.
In
July 2024, officers of the company provided a short term promissory note to the Company for $265,000
Schedule of Relatives or Related Parties to Directors Purchased Securities
|
Note
9 Related Party Transactions
The
Company had entered into an Exclusive and Commercial agreement with Kesin Pharma Corporation (“Kesin”), in which one of the
company’s board member is the President and CEO, and had a significant influence in the decision making, which makes it a related
party. Sales made to Kesin as a part of milestone structure for the years ended December 31,2023 and 2022 amounted to $800,000 and $300,000
respectively.
The
Company has leased its office from Saptalis Pharmaceuticals LLC (“Saptalis”) , in which one of the Company’s Director
is the President and CEO. Lease payments made during the year ended December 31, 2023 and 2022 are $ 7200 and $0 respectively. The company
has also engaged Saptalis to provide development services and conduct testing and studies for the products under development by the Company.
Expenses incurred towards such testing and studies which is included in the Research and Development Expenses in the Statement of Comprehensive
Loss for the year ended December 31, 2023 and December 31,2022 amounted to $355,124 and $647,566 respectively.
Relatives
or related parties to directors purchased securities from the Company on the same terms as unrelated parties as set forth below:
Schedule of Relatives or Related
Parties to Directors Purchased Securities
| Name of the Related
Party | |
Nature of transaction | |
Transactions
during the year ended
December 31 | | |
Balances
as at
December 31 | |
| | |
| |
2023 | | |
2022 | | |
2022 | | |
2023 | |
| Ms. Pushpa Shankar | |
Issue of Preferred Stock | |
$ | - | | |
$ | - | | |
$ | 750,000 | | |
$ | 750,000 | |
| Ms. Pushpa Shankar | |
Issue of Convertible notes | |
| 400,000 | | |
| 150,000 | | |
| 550,000 | | |
| 400,000 | |
| Ms. Yogita Desai | |
Issue of Preferred Stock | |
| - | | |
| - | | |
| 500,000 | | |
| 500,000 | |
| Ms. Yogita Desai | |
Issue of Convertible notes | |
| - | | |
| - | | |
| 100,000 | | |
| 100,000 | |
| Mr. Sandeep Gupta | |
Issue of Convertible notes | |
| - | | |
| 50,000 | | |
| 50,000 | | |
| - | |
| Total | |
| |
$ | 400,000 | | |
$ | 200,000 | | |
$ | 1,950,000 | | |
$ | 1,750,000 | |
|
| X |
- DefinitionThe entire disclosure for related party transactions. Examples of related party transactions include transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners; and (d) affiliates. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-5
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480990/946-20-50-6
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 235
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477968/946-235-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(g)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(c))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(2)(e))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/850/tableOfContent
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-6
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 850
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483326/850-10-50-1
| Name: |
us-gaap_RelatedPartyTransactionsDisclosureTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Scienture Inc. 2020 Stock Option and Grant Plan
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Scienture Inc. 2020 Stock Option and Grant Plan |
Note
10 Scienture Inc. 2020 Stock Option and Grant Plan
The
Stock Option and Grant Plan allows for the issuance of incentive stock options (“ISOs”), non-statutory stock options (“NSOs”).
ISOs may be granted only to the Company’s employees (including officers and directors who are also considered employees) and ex-employees.
NSOs may be granted to the Company’s employees and service providers such as advisors etc. Options under the Stock Option and Grant
Plan have a contractual term of not more than 10 years.
A
summary of the Company’s stock option activity under the Plans is as follows:
Summary of Stock Option Activity
| | |
Outstanding
Options | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Remaining
Term
(Years) | |
| Balance as of December 31, 2023 | |
| 655,000 | | |
$ | 0.90 | | |
| 7.56 | |
| Granted | |
| 142,199 | | |
| 1.13 | | |
| | |
| Exercised | |
| - | | |
| - | | |
| | |
| Cancelled and forfeited | |
| - | | |
| - | | |
| | |
| Balance as of June 20, 2024 | |
| 797,199 | | |
$ | 0.94 | | |
| 6.21 | |
| | |
| | | |
| | | |
| | |
| Vested and exercisable as of June 30, 2024 | |
| 499,089 | | |
$ | 0.84 | | |
| 5.62 | |
The
weighted-average grant date fair value of options granted during the six months ended June 30, 2024 was $0.76 per share.
The
Company recorded stock-based compensation expense in the Statement of Operations and Comprehensive Loss for the periods presented as
follows:
Schedule of Stock-based Compensation Expense
| | |
2024 | | |
2023 | |
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| General and Administrative Expenses | |
$ | 44,837 | | |
$ | 39,724 | |
| Total stock-based compensation expense | |
$ | 44,837 | | |
$ | 39,724 | |
Stock
Option Valuation Assumptions
The
fair value of each employee option grant was estimated on the date of grant using the Black-Scholes option pricing model and the following
assumptions for the periods indicated:
Schedule of Stock Option Valuation Assumptions
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Expected volatility | |
| 72 | % | |
| 65%
- 75 | % |
| Risk-free interest rate | |
| 4.1%
- 4.4 | % | |
| 0.5%
- 3.5 | % |
| Expected term | |
| 5.7
- 6.1 years | | |
| 5.9
- 6.0 years | |
| Expected dividend | |
| 0 | % | |
| 0 | % |
|
Note
10 Scienture Inc. 2020 Stock Option and Grant Plan
The
Stock Option and Grant Plan allows for the issuance of incentive stock options (“ISOs”), non-statutory stock options (“NSOs”).
ISOs may be granted only to the Company’s employees (including officers and directors who are also considered employees) and ex-employees.
NSOs may be granted to the Company’s employees and service providers such as advisors etc. Options under the Stock Option and Grant
Plan have a contractual term of not more than 10 years.
A
summary of the Company’s stock option activity under the Plans is as follows:
Summary of Stock Option Activity
| | |
Outstanding
Options | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Remaining
Term
(Years) | |
| Balance as of December 31, 2022 | |
| 560,000 | | |
$ | 0.83 | | |
| 7.95 | |
| Granted | |
| 185,000 | | |
| 1.13 | | |
| | |
| Exercised | |
| - | | |
| - | | |
| | |
| Cancelled and forfeited | |
| (90,000 | ) | |
| - | | |
| | |
| Balance as of December 31, 2023 | |
| 655,000 | | |
$ | 0.90 | | |
| 7.56 | |
| | |
| | | |
| | | |
| | |
| Vested and exercisable as of December 31, 2023 | |
| 410,065 | | |
$ | 0.84 | | |
| 6.84 | |
The
weighted-average grant date fair value of options granted during the years ended December 31, 2023 and 2022 are $ 0.55 and $ 0.52 per
share respectively.
The
Company recorded stock-based compensation expense in the Statement of Operations and Comprehensive Loss for the periods presented as
follows:
Schedule of Stock-based Compensation Expense
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| General and Administrative Expenses | |
$ | 79,449 | | |
$ | 68,087 | |
| Total stock-based compensation expense | |
$ | 79,449 | | |
$ | 68,087 | |
Stock
Option Valuation Assumptions
The
fair value of each employee option grant was estimated on the date of grant using the Black-Scholes option pricing model and the following
assumptions for the periods indicated:
Schedule of Stock Option Valuation Assumptions
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Expected volatility | |
| 65%
- 75 | % | |
| 65%
- 75 | % |
| Risk-free interest rate | |
| 0.5%
- 3.5 | % | |
| 0.5%
- 2.8 | % |
| Expected term | |
| 5.9
- 6.0 years | | |
| 5.9
- 6.0 years | |
| Expected dividend | |
| 0 | % | |
| 0 | % |
|
| X |
- DefinitionThe entire disclosure for share-based payment arrangement. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/718/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (h)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (l)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfCompensationRelatedCostsShareBasedPaymentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Warrants
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Warrants |
Note
11 Warrants
As
of June 30, 2024, there were 509,014 warrants outstanding and exercisable with an exercise price of $0.01 per share. The warrants were
granted in connection with the NVK debt (Refer - Note 6 - Long-Term Convertible Debt, net of debt discount).
Summary of Assumptions Used to Estimate Fair Value of Warrants
|
Note
11 Warrants
As
of December 31, 2023, there were 509,014 warrants outstanding and exercisable with an exercise price of $0.01 per share. The warrants
were granted in connection with the NVK debt (Refer - Note 6 - Long-Term Convertible Debt, net of debt discount).
The
following table summarizes the assumptions used to estimate the fair value of the outstanding warrants during the years ended December
31, 2023, and 2022:
Summary
of Assumptions Used to Estimate Fair Value of Warrants
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Expected dividend yield | |
| 0 | % | |
| - | |
| Weighted-average expected volatility | |
| 70.52 | % | |
| - | |
| Weighted-average risk-free interest rate | |
| 4.50 | % | |
| - | |
| Expected life of warrants | |
| 5
years | | |
| - | |
|
| X |
- Definition
+ References
+ Details
| Name: |
SCNX_WarrantsTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Net Loss per Share
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Net Loss per Share |
Note
12 Net Loss per Share
Stock
options to purchase 799,199 and 655,000 shares of common stock, warrants to purchase 509,014 and 0 common stock, convertible preferred
stock and convertible notes to purchase 3,365,669 and 3,195,911 common stock and long-term convertible debt to purchase 0 and 3,350,000
shares common stock were outstanding at June 30, 2024 and 2023, respectively, that were not included in the computation of diluted weighted
average common shares outstanding because their effect would have been anti-dilutive.
|
Note
12 Net Loss per Share
Stock
options to purchase 655,000 and 560,000 common stock, warrants to purchase 509,014 and 0 common stock, convertible preferred stock and
convertible notes to purchase 3,365,669 and 3,195,911 common stock and long-term convertible debt to purchase 9,529,683 and 0 common
stock were outstanding at December 31, 2023 and 2022, respectively, that were not included in the computation of diluted weighted average
common shares outstanding because their effect would have been anti-dilutive.
|
| X |
- DefinitionThe entire disclosure for earnings per share. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/260/tableOfContent
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-3
| Name: |
us-gaap_EarningsPerShareTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Leases
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Leases |
Note
13 Leases
The
Company determines if an arrangement is a lease at inception. Operating leases are included in Operating lease, Right of Use asset, Operating
Lease Liability (Current and Non-Current) in the Company’s balance sheets. The ROU assets represent the Company’s right to
use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising
from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments
over the lease term. As most of the leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate
at commencement date. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain
that the Company will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
As a practical expedient, the Company elected, for all office and facility leases, not to separate non-lease components from lease components
and instead to account for each separate lease component and its associated non-lease components as a single lease component. The Company
made an accounting policy election by class of underlying asset not to recognize the lease liability and related right-of-use asset for
leases with a term of one year or less.
The
Company has an operating lease for administrative office. The lease has remaining lease term around three years.
The
components of lease expense were as follows:
Schedule
of Lease Expense
| | |
| | | |
| | |
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Operating lease costs | |
| | | |
| | |
| Amortization of ROU Assets | |
$ | 2,152 | | |
$ | - | |
| Interest on Lease Liabilities | |
$ | 1,190 | | |
$ | - | |
| Short term lease costs | |
$ | 17,010 | | |
$ | - | |
Supplemental
balance sheet information related to leases was as follows:
Schedule
of Supplemental Balance Sheet Information Related To Leases
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Operating Leases | |
| | | |
| | |
| Right of Use Assets | |
$ | 61,579 | | |
$ | 64,091 | |
| | |
| | | |
| | |
| Short term Lease liabilities | |
$ | 22,567 | | |
$ | 21,404 | |
| Long term Lease liabilities | |
$ | 39,319 | | |
$ | 42,893 | |
| Total Lease Liabilities | |
$ | 61,886 | | |
$ | 64,297 | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term (in years) | |
| 2.33 | | |
| 2.83 | |
| | |
| | | |
| | |
| Weighted Average Discount Rate | |
| 15.50 | % | |
| 15.50 | % |
|
Note
13 Leases
The
Company determines if an arrangement is a lease at inception. Operating leases are included in Operating lease, Right of Use asset, Operating
Lease Liability (Current and Non-Current) in the Company’s balance sheets. The ROU assets represent the Company’s right to
use an underlying asset for the lease term and lease liabilities represent the Company’s obligation to make lease payments arising
from the lease. Operating lease ROU assets and liabilities are recognized at commencement date based on the present value of lease payments
over the lease term. As most of the leases do not provide an implicit rate, the Company generally uses its incremental borrowing rate
at commencement date. The Company’s lease terms may include options to extend or terminate the lease when it is reasonably certain
that the Company will exercise that option. Lease expense for lease payments is recognized on a straight-line basis over the lease term.
As a practical expedient, the Company elected, for all office and facility leases, not to separate non-lease components from lease components
and instead to account for each separate lease component and its associated non-lease components as a single lease component. The Company
made an accounting policy election by class of underlying asset not to recognize the lease liability and related right-of-use asset for
leases with a term of one year or less.
The
Company has an operating lease for administrative office. The lease has remaining lease term around three years.
The
components of lease expense were as follows:
Schedule
of Lease Expense
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Operating lease costs | |
| | | |
| | |
| Amortization of ROU Assets | |
$ | 5,025 | | |
$ | - | |
| Interest on Lease Liabilities | |
$ | 2,380 | | |
$ | - | |
| Short term lease costs | |
$ | 34,021 | | |
$ | 32,677 | |
Supplemental
cash flow information related to leases was as follows:
Schedule
of Supplemental Cash Flow Information Related To Leases
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Cash paid for accounts included in the measurements of lease liabilities | |
| | |
| |
| Operating cash flows for Operating leases | |
$ | 7,200 | | |
$ | - | |
| Right of Use Assets obtained in exchange for new Lease
Liabilities | |
$ | 205 | | |
$ | - | |
Supplemental
balance sheet information related to leases was as follows:
Schedule
of Supplemental Balance Sheet Information Related To Leases
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Operating Leases | |
| | | |
| | |
| Right of Use Assets | |
$ | 64,091 | | |
$ | - | |
| | |
| | | |
| | |
| Short term Lease liabilities | |
$ | 21,404 | | |
$ | - | |
| Long term Lease liabilities | |
$ | 42,893 | | |
$ | - | |
| Total Lease Liabilities | |
$ | 64,297 | | |
$ | - | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term (in years) | |
| 2.83 | | |
| - | |
| | |
| | | |
| | |
| Weighted Average Discount Rate | |
| 15.50 | % | |
| - | |
Maturities
of lease liabilities were as follows at December 31, 2023:
Schedule
of Maturities Of Lease Liabilities
| December 31, 2023 | |
| |
| 2024 | |
$ | 29,017 | |
| 2025 | |
| 29,887 | |
| 2026 | |
| 17,823 | |
| Total lease payments | |
| 76,727 | |
| Less: Imputed interest | |
| 12,430 | |
| Total | |
$ | 64,297 | |
|
| X |
- DefinitionThe entire disclosure for operating leases of lessee. Includes, but is not limited to, description of operating lease and maturity analysis of operating lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/842-20/tableOfContent
| Name: |
us-gaap_LesseeOperatingLeasesTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Subsequent Events
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Subsequent Events |
Note
14 Subsequent Events
In
July 2024, the executives of the Company issued a short-term loan to Company for an aggregate amount of $250,000.
In
July 2024, all of the unvested options per the Company’s Stock Option and Grant Plan became vested.
Business
Combination
On
July 25, 2024, Scienture, Inc. (the “Company”) entered into and closed an Agreement and Plan of Merger (the “Merger
Agreement”) with MEDS, MEDS Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of MEDS (“Merger Sub I”)
and MEDS Merger Sub II, LLC, a Delaware limited liability company and wholly owned subsidiary of the Company (“Merger Sub II”).
Pursuant to the Merger Agreement, (i) Merger Sub I merged with and into the Company, with the Company continuing as the surviving entity
and a wholly owned subsidiary of MEDS, and (ii) the Company merged with and into Merger Sub II, with Merger Sub II continuing as the
surviving entity.
Management
has evaluated subsequent events through August 15, 2024, the date the financial statements were available to be issued.
|
Note
14 Subsequent Events
Short-Term
Convertible Notes
All
the short-term convertible notes matured during the period of December 2023. The Company has not paid the amounts due including the principal
and the accrued interest. However, in March 2024, the Company had converted the outstanding principal of $3,665,220 and the accrued interest
till the date of conversion amounting to $276,233 into an aggregate of 965,568 preferred stock of the company.
Business
Combination
On
July 25, 2024, Scienture, Inc. (the “Company”) entered into and closed an Agreement and Plan of Merger (the “Merger
Agreement”) with MEDS, MEDS Merger Sub I, Inc., a Delaware corporation and wholly owned subsidiary of MEDS (“Merger Sub I”)
and MEDS Merger Sub II, LLC, a Delaware limited liability company and wholly owned subsidiary of the Company (“Merger Sub II”).
Pursuant to the Merger Agreement, (i) Merger Sub I merged with and into the Company, with the Company continuing as the surviving entity
and a wholly owned subsidiary of MEDS, and (ii) the Company merged with and into Merger Sub II, with Merger Sub II continuing as the
surviving entity.
Termination
of Exclusive License and Commercial Agreement:
Scienture
Inc. (Scienture) and Kesin had entered into two exclusive license commercial agreements where Scienture had granted Kesin the rights
to commercialize the products. In March 2024, the parties have terminated the agreement, and the parties agreed that, Scienture shall
pay Kesin a total gross amount of $1,285,000 upon commercialization of product via a royalty arrangement.
This
agreement also requires that if the full $1,285,000 has not been repaid within two years of the early of i) commercial launch or ii)
120 days from FDA approval, then interest will accrue prospectively at a rate of 8% annually on unpaid balance. As of the date of issue
of financial statements, the entire amount is outstanding.
Management
has evaluated subsequent events through July 31, 2024, the date the financial statements were available to be issued.
|
| X |
- DefinitionThe entire disclosure for significant events or transactions that occurred after the balance sheet date through the date the financial statements were issued or the date the financial statements were available to be issued. Examples include: the sale of a capital stock issue, purchase of a business, settlement of litigation, catastrophic loss, significant foreign exchange rate changes, loans to insiders or affiliates, and transactions not in the ordinary course of business. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-Name Accounting Standards Codification
-Publisher FASB
-URI https://asc.fasb.org/855/tableOfContent
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Summary of Significant Accounting Policies (Policies) - Scienture Inc [Member]
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Use of Estimates |
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S.GAAP requires the Company to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. These estimates are based on information available through the
date of the issuance of the financial statements and actual results could differ from those estimates. To the extent there are material
differences between the Company’s estimates and the actual results, the Company’s future consolidated results of operation
may be affected. Areas requiring significant estimates and assumptions by the Company include, but are not limited to:
| |
● |
fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| |
● |
accruals
for estimated liabilities; |
| |
● |
the
valuation of stock-based compensation awards ; and |
| |
● |
provisions
for income taxes and related valuation allowances and tax uncertainties. |
|
Use
of Estimates
The
preparation of the Company’s financial statements in conformity with U.S.GAAP requires the Company to make estimates and assumptions
that affect the reported amounts of certain assets and liabilities; the reported amounts of revenues and expenses for the periods covered
and certain amounts disclosed in the notes to the financial statements. These estimates are based on information available through the
date of the issuance of the financial statements and actual results could differ from those estimates. To the extent there are material
differences between the Company’s estimates and the actual results, the Company’s future consolidated results of operation
may be affected. Areas requiring significant estimates and assumptions by the Company include, but are not limited to:
| |
● |
fair
value of long-term convertible debt and warrants issued in connection with such debt; |
| |
● |
accruals
for estimated liabilities; |
| |
● |
lease
term |
| |
● |
the
valuation of stock-based compensation awards ; and |
| |
● |
provisions
for income taxes and related valuation allowances and tax uncertainties. |
|
| Unaudited Interim Financial Information |
Unaudited
Interim Financial Information
The
unaudited condensed consolidated interim financial statements and related notes have been prepared in accordance with U.S. GAAP for interim
financial information, within the rules and regulations of the United States Securities and Exchange Commission (the “SEC”).
Certain information and disclosures normally included in the annual financial statements prepared in accordance with U.S. GAAP have been
condensed or omitted pursuant to such rules and regulations. The unaudited interim financial statements have been prepared on a basis
consistent with the audited financial statements and in the opinion of management, reflect all adjustments, consisting of only normal
recurring adjustments, necessary for the fair presentation of the results for the interim periods presented and of the financial condition
as of the date of the interim balance sheet. The financial data and the other information disclosed in these notes to the interim financial
statements related to the six-month periods are unaudited. Unaudited interim results are not necessarily indicative of the results for
the full fiscal year.
The
accompanying unaudited interim condensed consolidated financial statements should be read in conjunction with the Company’s audited
financial statements and the notes thereto for the year ended December 31, 2023.
|
|
| Liquidity |
Liquidity
The
entity has just commenced operations and is expected to be funded by the stockholders for liquidity purposes. The liquidity position
of the entity is also dependent on the fundings by the additional development partners.
|
Liquidity
The
entity has just commenced operations and is expected to be funded by the stockholders for liquidity purposes. The liquidity position
of the entity is also dependent on the fundings by the additional development partners.
|
| Comprehensive Loss |
Comprehensive
Loss
Comprehensive
loss includes net loss as well as other changes in stockholder’s equity that result from transactions and economic events other
than those with stockholders. There was no difference between net loss and comprehensive loss presented in the financial statements for
the six months ended June 30, 2024 and 2023.
|
Comprehensive
Loss
Comprehensive
loss includes net loss as well as other changes in stockholder’s equity that result from transactions and economic events other
than those with stockholders. There was no difference between net loss and comprehensive loss presented in the financial statements for
the years ended December 31, 2023 and 2022.
|
| Segment Reporting |
Segment
Reporting
The
Company’s chief operating decision-maker is its Chief Executive Officer, who makes resource allocation decisions and assesses performance
based on financial information presented on an aggregate basis. There are no segment managers who are held accountable by the chief operating
decision-maker, or anyone else, for any planning, strategy and key decision-making regarding operations. Accordingly, the Company has
a single reportable segment and operating segment structure.
|
Segment
Reporting
The
Company’s chief operating decision-maker is its Chief Executive Officer, who makes resource allocation decisions and assesses performance
based on financial information presented on an aggregate basis. There are no segment managers who are held accountable by the chief operating
decision-maker, or anyone else, for any planning, strategy and key decision-making regarding operations. Accordingly, the Company has
a single reportable segment and operating segment structure.
|
| Cash and Cash Equivalents |
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash
equivalents. Cash equivalents consist of amounts invested in money market funds and are stated at fair value.
|
Cash
and Cash Equivalents
The
Company considers all highly liquid investments with an original maturity of three months or less from the date of purchase to be cash
equivalents. Cash equivalents consist of amounts invested in money market funds and are stated at fair value.
|
| Accounts Receivable |
Accounts
Receivable
Accounts
receivable consist of milestone payments due from development partners as a consideration for the rights granted for the commercialization
of the products to be developed. The Company reviews its accounts receivable and provides allowances of specific amounts if collectability
is no longer reasonably assured based on historical experience and specific collection issues. The allowance for doubtful accounts was
$0 as of June 30, 2024 and December 31, 2023, respectively.
|
Accounts
Receivable
Accounts
receivable consist of milestone payments due from development partners as a consideration for the rights granted for the commercialization
of the products to be developed. The Company reviews its accounts receivable and provides allowances of specific amounts if collectability
is no longer reasonably assured based on historical experience and specific collection issues. The allowance for doubtful accounts was
$0 as of December 31, 2023 and 2022, respectively.
(Refer
– Note 14– Subsequent Events – Termination of Exclusive License and Commercial Agreement).
|
| Revenue Recognition |
Revenue
Recognition
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services.
The
Company adopted FASB ASU No. 2014-09, Revenue from Contracts with Customers and the related amendments, which are codified into ASC 606,
which establishes a broad principle that requires entities to assess the products or services promised in contracts with customers at
contract inception to determine the appropriate unit at which to record revenues, which is referred to as a performance obligation. Revenue
is recognized when control of the promised products or services is transferred to customers, at an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those products or services.
To
determine revenue recognition for arrangements that the Company determines are within the scope of ASC 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii)
determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it
is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the
customer. At contract inception, once the contract was determined to be within the scope of ASC 606, the Company assessed the goods or
services promised within each contract and determined those that were performance obligations, and assessed whether each promised good
or service was distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective
performance obligation when (or as) the performance obligation is satisfied.
A
performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in
ASC 606. The Company recognizes revenue at the point of sale of service.
|
Revenue
Recognition
Revenue
is recognized when control of the promised services is transferred to customers, at an amount that reflects the consideration to which
the entity expects to be entitled in exchange for those services.
The
Company adopted FASB ASU No. 2014-09, Revenue from Contracts with Customers and the related amendments, which are codified into ASC 606,
which establishes a broad principle that requires entities to assess the products or services promised in contracts with customers at
contract inception to determine the appropriate unit at which to record revenues, which is referred to as a performance obligation. Revenue
is recognized when control of the promised products or services is transferred to customers, at an amount that reflects the consideration
to which the entity expects to be entitled in exchange for those products or services.
To
determine revenue recognition for arrangements that the Company determines are within the scope of ASC 606, the Company performs the
following five steps: (i) identify the contract(s) with a customer; (ii) identify the performance obligation(s) in the contract; (iii)
determine the transaction price; (iv) allocate the transaction price to the performance obligation(s) in the contract; and (v) recognize
revenue when (or as) the entity satisfies a performance obligation. The Company only applies the five-step model to contracts when it
is probable that the entity will collect the consideration it is entitled to in exchange for the goods or services it transfers to the
customer. At contract inception, once the contract was determined to be within the scope of ASC 606, the Company assessed the goods or
services promised within each contract and determined those that were performance obligations, and assessed whether each promised good
or service was distinct. The Company then recognizes as revenue the amount of the transaction price that is allocated to the respective
performance obligation when (or as) the performance obligation is satisfied.
A
performance obligation is a promise in a contract to transfer a distinct good or service to the customer and is the unit of account in
ASC 606. The Company recognizes revenue at the point of sale of service.
|
| Exclusive License and Commercial Agreements |
Exclusive
License and Commercial Agreements
The
Company entered into an exclusive license and commercial agreement with Kesin Pharma Corporation, a related party where the Company granted
the exclusive license rights to commercialize SCN-102 in 2022 and SCN-104 in 2023 to Kesin (SCN-102 and SCN-104 are together referred
to as “the Products”) for use in the United States of America. In consideration of the rights granted, the Company is in
receipt of milestone payments and reimbursement of costs actually incurred related to the products. Revenue has been recognized when
such development milestone events take place and the amounts are due to be received. The Company recognized $800,000 for the six months
ended June 30, 2023, at the point when the development milestone events occurred.
In
March 2024, the parties have terminated the agreement, and the parties agreed that, Scienture shall pay Kesin a total gross amount of
$1,285,000 upon commercialization of product via a royalty arrangement.
This
agreement also requires that if the full $1,285,00 has not been repaid within two years of the early of i) commercial launch or ii) 120
from FDA approval, then interest will accrue prospectively at a rate of 8% annually on unpaid balance. Accordingly, the Company recorded
a $1,285,000 termination fee liability. As of the date of issue of financial statements, the entire amount is outstanding.
|
Exclusive
License and Commercial Agreements
The
Company entered into an exclusive license and commercial agreement with Kesin Pharma Corporation, a related party where the Company granted
the exclusive license rights to commercialize SCN-102 in 2022 and SCN-104 in 2023 to Kesin (SCN-102 and SCN-104 are together referred
to as “the Products”) for use in the United States of America. In consideration of the rights granted, the Company is in
receipt of milestone payments and reimbursement of costs actually incurred related to the products. Revenue has been recognized when
such development milestone events take place and the amounts are due to be received. The Company recognized $800,000 and $300,000, respectively,
during the years ended December 31, 2023 and 2022 at the point when the development milestone events occurred. (Refer – Note 14–
Subsequent Events – Termination of Exclusive License and Commercial Agreement).
|
| Fair Value of Financial Instruments |
Fair
Value of Financial Instruments
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes
the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available.
Observable inputs are inputs that market participants would use in pricing the asset or liability and are developed based on market data
obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions of what
market participants would use in pricing the asset or liability based on the best information available in the circumstances. The financial
and nonfinancial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurement.
The hierarchy is presented down into three levels based on the reliability of the inputs.
| |
Level
1 |
Quoted
prices are available in active markets for identical assets or liabilities. |
| |
|
|
| |
Level
2 |
Observable
inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets
or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially
the full term of the assets or liabilities. |
| |
|
|
| |
Level
3 |
Unobservable
pricing inputs that are generally less observable from objective sources, such as discounted cash flow models or valuations. |
The
carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term convertible notes approximate their
fair value because of the short-term nature of these instruments. The carrying amount of long-term convertible debt approximate the fair
value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
|
Fair
Value of Financial Instruments
Fair
value is defined as the price that would be received to sell an asset or paid to transfer a liability (an exit price) in an orderly transaction
between market participants at the measurement date. A hierarchy has been established for inputs used in measuring fair value that maximizes
the use of observable inputs and minimizes the use of unobservable inputs by requiring that the most observable inputs be used when available.
Observable inputs are inputs that market participants would use in pricing the asset or liability and are developed based on market data
obtained from sources independent of the Company. Unobservable inputs are inputs that reflect the Company’s assumptions of what
market participants would use in pricing the asset or liability based on the best information available in the circumstances. The financial
and nonfinancial assets and liabilities are classified based on the lowest level of input that is significant to the fair value measurement.
The hierarchy is presented down into three levels based on the reliability of the inputs.
| |
Level
1 |
Quoted
prices are available in active markets for identical assets or liabilities. |
| |
|
|
| |
Level
2 |
Observable
inputs other than quoted prices in active markets for identical assets and liabilities, quoted prices for identical or similar assets
or liabilities in inactive markets, or other inputs that are observable or can be corroborated by observable market data for substantially
the full term of the assets or liabilities. |
| |
|
|
| |
Level
3 |
Unobservable
pricing inputs that are generally less observable from objective sources, such as discounted cash flow models or valuations. |
The
carrying amounts of cash, accounts receivable, accounts payable, accrued liabilities and short-term convertible notes approximate their
fair value because of the short-term nature of these instruments. The carrying amount of long-term convertible debt approximate the fair
value because the debt is based on current rates at which the Company could borrow funds with similar maturities.
|
| Concentration of Credit Risks and Major Customers |
Concentration
of Credit Risks and Major Customers
Financial
instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The
Company places its cash and cash equivalents with financial institutions. During the six months ended June 30, 2024, the Company had
one development partner that accounted for the entire revenue recognized in the Statement of Comprehensive Loss.
|
Concentration
of Credit Risks and Major Customers
Financial
instruments that potentially subject the Company to credit risk consist principally of cash and cash equivalents and receivables. The
Company places its cash and cash equivalents with financial institutions. During the years ended December 31, 2023, and 2022, the company
had one development partner that accounted for the entire revenue recognized in the Statement of Comprehensive Loss.
|
| Research & Development Expenses |
Research
& Development Expenses
Research
and development costs are expensed in the period incurred in accordance with ASC 730. Research and development expenses consist of independent
contractor costs , costs for outsourced analytical research and development activities, batch manufacturing cost and, advisory costs
as a part of research, market research costs and other regulatory consulting costs.
|
Research
& Development Expenses
Research
and development costs are expensed in the period incurred in accordance with ASC 730. Research and development expenses consist of independent
contractor costs , costs for outsourced analytical research and development activities, batch manufacturing cost and, advisory costs
as a part of research, market research costs and other regulatory consulting costs.
|
| Stock-Based Compensation |
Stock-Based
Compensation
The
Company’s stock-based compensation expense relates to stock options. Stock-based compensation expense for its stock-based awards
is based on their grant date fair value. The Company estimates the fair value of stock option awards on the grant date using the Black-Scholes
option-pricing model. The Black-Scholes model considers several variables and assumptions in estimating the fair value of stock-based
awards. These variables include the per share fair value of the underlying common stock, exercise price, expected term, risk-free interest
rate, expected annual dividend yield and the expected stock price volatility over the expected term. The Company has estimated volatility
by reference to the historical volatilities of the Company and that of similar publicly traded peer companies. The risk-free interest
rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration to the expected term of the equity-settled
award.
|
Stock-Based
Compensation
The
Company’s stock-based compensation expense relates to stock options. Stock-based compensation expense for its stock-based awards
is based on their grant date fair value. The fair values of stock-based compensations are recognized as compensation expense on a straight-line
basis over the requisite service period in which the awards are expected to vest. The Company estimates the fair value of stock option
awards on the grant date using the Black-Scholes option-pricing model. The Black-Scholes model considers several variables and assumptions
in estimating the fair value of stock-based awards. These variables include the per share fair value of the underlying common stock,
exercise price, expected term, risk-free interest rate, expected annual dividend yield and the expected stock price volatility over the
expected term. The Company has estimated volatility by reference to the historical volatilities of the Company and that of similar publicly
traded peer companies. The risk-free interest rate is based on the yield available on U.S. Treasury zero-coupon issues similar in duration
to the expected term of the equity-settled award.
|
| Warrant Valuation |
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
The Company accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
|
Warrant
Valuation
Stock
warrant valuation models require the input of highly subjective assumptions. The fair value of stock-based payment awards is estimated
using the Black-Scholes option model with a volatility figure derived from an average of historical stock prices for comparable entities.
The Company accounts for the expected life based on the contractual life of the warrants. The risk-free interest rate is determined from
the implied yields of U.S. Treasury zero-coupon bonds with a remaining life consistent with the expected term of the warrants.
|
| Net Loss per share |
Net
Loss per share
Basic
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period,
adjusted for outstanding shares that are subject to repurchase.
For
the calculation of diluted net loss per share, basic net loss per share is adjusted by the effect of dilutive securities if any. Diluted
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding and potential
common stock outstanding, if dilutive. For periods in which the Company reports net losses, diluted net loss per share is the same as
basic net loss per share because potentially dilutive shares of common stock are not assumed to have been issued if their effect is anti-dilutive.
Disclosure - Schedule of Deferred Tax Assets and Related Valuation Allowance (Details)
|
Net
Loss per share
Basic
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding during the period,
adjusted for outstanding shares that are subject to repurchase.
For
the calculation of diluted net loss per share, basic net loss per share is adjusted by the effect of dilutive securities if any. Diluted
net loss per share is computed by dividing net loss by the weighted-average number of shares of common stock outstanding and potential
common stock outstanding, if dilutive. For periods in which the Company reports net losses, diluted net loss per share is the same as
basic net loss per share because potentially dilutive shares of common stock are not assumed to have been issued if their effect is anti-dilutive.
Recent
Accounting Pronouncements
|
| Accounting Pronouncements Not Yet Adopted |
Accounting
Pronouncements Not Yet Adopted
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2023-07, “Segment
Reporting (Topic 280): Improvements to Reportable Segment Disclosures” (“ASU 2023-07”), which requires additional operating
segment disclosures in annual and interim consolidated financial statements. ASU 2023-07 is effective for annual periods beginning after
December 15, 2023 and for interim periods beginning after December 15, 2024 on a retrospective basis, with early adoption permitted.
The Company is evaluating the effect of adopting ASU 2023-07.
In
December 2023, the FASB issued Accounting Standards Update No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”
(“ASU 2023-09”), which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components
of the effective tax rate reconciliation and modifies other income tax-related disclosures. ASU 2023-09 is effective for annual periods
beginning after December 15, 2024 on a retrospective or prospective basis. The Company is evaluating the effect of adopting ASU 2023-09.
|
Accounting
Pronouncements Not Yet Adopted
In
November 2023, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2023-07, “Segment
Reporting (Topic 280): Improvements to Reportable Segment Disclosures” (“ASU 2023-07”), which requires additional operating
segment disclosures in annual and interim consolidated financial statements. ASU 2023-07 is effective for annual periods beginning after
December 15, 2023 and for interim periods beginning after December 15, 2024 on a retrospective basis, with early adoption permitted.
The Company is evaluating the effect of adopting ASU 2023-07.
In
December 2023, the FASB issued Accounting Standards Update No. 2023-09, “Income Taxes (Topic 740): Improvements to Income Tax Disclosures”
(“ASU 2023-09”), which requires disclosure of disaggregated income taxes paid, prescribes standard categories for the components
of the effective tax rate reconciliation and modifies other income tax-related disclosures. ASU 2023-09 is effective for annual periods
beginning after December 15, 2024 on a retrospective or prospective basis. The Company is evaluating the effect of adopting ASU 2023-09.
|
| Income Taxes |
|
Income
Taxes
State
Income Tax:
The
Company is incorporated in Delaware and headquartered in New York where the state tax is 8.70% and 7.25% respectively. However, due to
losses for the years ended December 31, 2023 and 2022, no provision on state income tax has been recognized.
Federal
Income Tax:
The
Company is a C Corporation for tax purposes, filing Form 1120 annually. Profits are not being passed through to owners. The company records
income taxes pursuant to the liability method. The Company has a loss before tax of ($2,240,989) and ($3,708,378) for years ended December
31, 2023 and 2022 respectively. Therefore, no provision for federal income tax has been recognized.
|
| Deferred Tax Assets and Liabilities |
|
Deferred
Tax Assets and Liabilities
Deferred
tax assets and liabilities are determined based on the differences between the financial statement and the tax basis of assets and liabilities.
Realization of the future tax benefits related to the net deferred tax assets is dependent on many factors including the Company’s
ability to generate taxable income. Management believes that, at a minimum, it is more likely than not that future taxable income may
not be sufficient to realize the recorded assets.
The
Company has recorded a deferred tax asset related to its net operating loss carryforwards, timing difference between written down value
of assets, and unutilized R&D credit, which are expected to reduce future taxable income. The company has assessed the likelihood
of realizing the deferred tax assets and determined that it is more likely than not that a portion of the assets may not be realized.
Therefore, a valuation allowance has been created to account for 100% of the deferred tax assets to its expected realizable value.
The
impact of the deferred tax assets and related valuation allowance on the Company’s financial statements is as follows:
Schedule
of Deferred Tax Assets and Related Valuation Allowance
| | |
2023 | | |
2022 | |
| | |
Year
Ended December 31, | |
| | |
2023 | | |
2022 | |
| Deferred Tax Assets | |
| | | |
| | |
| Net operating loss carryforwards | |
$ | 3,173,840 | | |
$ | 2,491,667 | |
| Research and development tax credits | |
| 6,635 | | |
| 2,705 | |
| Property and equipment and operating lease liability | |
| 49,220 | | |
| 53,960 | |
| Valuation allowance | |
| (3,229,695 | ) | |
| (2,548,331 | ) |
| Net deferred tax assets | |
$ | - | | |
$ | - | |
|
| Accounting Pronouncements Recently Adopted |
|
Accounting
Pronouncements Recently Adopted
The
Company has implemented all new relevant accounting pronouncements that are in effect through the date of these financial statements.
The pronouncements did not have any material impact on the financial statements unless otherwise disclosed, and the Company does not
believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial
position or results of operations.
In
June 2016, the FASB issued ASU No. 2016-13, Financial Instruments—Credit Losses (ASC 326), which provides guidance on measurement
of credit losses on financial instruments. This ASU adds a current expected credit loss impairment model to U.S.GAAP that is based on
expected losses rather than incurred losses whereby a broader range of reasonable and supportable information is required to be utilized
in order to derive credit loss estimates. The effective date of the new guidance as amended by ASU No. 2019-10 is fiscal years beginning
after December 15, 2022, including interim periods within those fiscal years. The Company adopted ASU 2016-13 effective January 1, 2023
the company determined that the update applied to trade receivables, but that there no material impact to the financial statements from
the adoption of ASU 2016-13.
In
February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standard Update (“ASU”) No.
2016-2, Leases, to provide guidance for the accounting for leasing transactions. The standard requires the lessee to recognize a lease
liability along with a right-of-use asset for all leases with a term longer than one year. A lessee is permitted to make an accounting
policy election by class of underlying asset to not recognize the lease liability and related right-of-use asset for leases with a term
of one year or less. The provisions of this standard also apply to situations where the Company is the lessor. In March 2019, the FASB
issued ASU 2019-01, “Lease (842): Codification improvements.” This updated clarified that entities were exempt from disclosing
the effect of the change on income from continuing operations, net income, and related per-share amounts, if applicable, for interim
periods after the adoption of Accounting Standards Codification (“ASC”) 842.
The
standard was initially effective for annual and interim reporting periods beginning after December 15, 2019. However, in November 2019,
the FASB issued ASU 2019-10, “Financial Instruments Credit Losses (Topic 326), Derivatives and Hedging (Topic 815), and Leases
(Topic 842): Effective Dates”, which deferred the effective date of ASU 2016-02 by an additional year. At its April 8, 2020, meeting,
the FASB voted to defer the effective date for ASC 842 another year. As such, the Company is required to adopt the new leases standard
for fiscal years beginning after December 15, 2021, and interim periods within fiscal years beginning after December 15, 2022. The Company
adopted this new guidance effective January 1, 2022.
|
| X |
- DefinitionAccounting Pronouncements Not Yet Adopted [Policy Text Block]
| Name: |
SCNX_AccountingPronouncementsNotYetAdoptedPolicyTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDeferred Tax Assets and Liabilities [Policy Text Block]
| Name: |
SCNX_DeferredTaxAssetsAndLiabilitiesPolicyTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExclusive License and Commercial Agreements [Policy Text Block]
| Name: |
SCNX_ExclusiveLicenseAndCommercialAgreementsPolicyTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLiquidity [Policy Text Block]
| Name: |
SCNX_LiquidityPolicyTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionUnaudited Interim Financial Information [Policy Text Block]
| Name: |
SCNX_UnauditedInterimFinancialInformationPolicyTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWarrant Valuation [Policy Text Block]
| Name: |
SCNX_WarrantValuationPolicyTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for cash and cash equivalents, including the policy for determining which items are treated as cash equivalents. Other information that may be disclosed includes (1) the nature of any restrictions on the entity's use of its cash and cash equivalents, (2) whether the entity's cash and cash equivalents are insured or expose the entity to credit risk, (3) the classification of any negative balance accounts (overdrafts), and (4) the carrying basis of cash equivalents (for example, at cost) and whether the carrying amount of cash equivalents approximates fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482913/230-10-50-1
| Name: |
us-gaap_CashAndCashEquivalentsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for salaries, bonuses, incentive awards, postretirement and postemployment benefits granted to employees, including equity-based arrangements; discloses methodologies for measurement, and the bases for recognizing related assets and liabilities and recognizing and reporting compensation expense. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_CompensationRelatedCostsPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for comprehensive income.
| Name: |
us-gaap_ComprehensiveIncomePolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for credit risk. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 825
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478898/942-825-50-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
| Name: |
us-gaap_ConcentrationRiskCreditRisk |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for computing basic and diluted earnings or loss per share for each class of common stock and participating security. Addresses all significant policy factors, including any antidilutive items that have been excluded from the computation and takes into account stock dividends, splits and reverse splits that occur after the balance sheet date of the latest reporting period but before the issuance of the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 260
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-2
| Name: |
us-gaap_EarningsPerSharePolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for determining the fair value of financial instruments. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 825
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-1
| Name: |
us-gaap_FairValueOfFinancialInstrumentsPolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for income taxes, which may include its accounting policies for recognizing and measuring deferred tax assets and liabilities and related valuation allowances, recognizing investment tax credits, operating loss carryforwards, tax credit carryforwards, and other carryforwards, methodologies for determining its effective income tax rate and the characterization of interest and penalties in the financial statements. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 20
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-20
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 19
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-19
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 25
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482525/740-10-45-25
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-03(h)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479886/946-10-S99-3
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 17
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-17
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-9
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482525/740-10-45-28
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-1
| Name: |
us-gaap_IncomeTaxPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy pertaining to new accounting pronouncements that may impact the entity's financial reporting. Includes, but is not limited to, quantification of the expected or actual impact.
| Name: |
us-gaap_NewAccountingPronouncementsPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for costs it has incurred (1) in a planned search or critical investigation aimed at discovery of new knowledge with the hope that such knowledge will be useful in developing a new product or service, a new process or technique, or in bringing about a significant improvement to an existing product or process; or (2) to translate research findings or other knowledge into a plan or design for a new product or process or for a significant improvement to an existing product or process. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 05
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483044/730-10-05-1
| Name: |
us-gaap_ResearchAndDevelopmentExpensePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for revenue. Includes revenue from contract with customer and from other sources. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (e)
-SubTopic 10
-Topic 235
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483426/235-10-50-4
| Name: |
us-gaap_RevenueRecognitionPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for segment reporting. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (bb)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 54
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-54
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 36
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-36
Reference 6: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 29
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-29
| Name: |
us-gaap_SegmentReportingPolicyPolicyTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for accounts receivable. Reference 1: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-6
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-2
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 310
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481569/310-20-50-1
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 15
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-15
Reference 6: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-11B
| Name: |
us-gaap_TradeAndOtherAccountsReceivablePolicy |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionDisclosure of accounting policy for the use of estimates in the preparation of financial statements in conformity with generally accepted accounting principles. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-9
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-4
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-11
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 12
-SubTopic 10
-Topic 275
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-12
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 275
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482861/275-10-50-8
| Name: |
us-gaap_UseOfEstimates |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Summary of Significant Accounting Policies (Tables)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Schedule of Deferred Tax Assets and Related Valuation Allowance |
Disclosure - Schedule of Deferred Tax Assets and Related Valuation Allowance (Details)
|
The
impact of the deferred tax assets and related valuation allowance on the Company’s financial statements is as follows:
Schedule
of Deferred Tax Assets and Related Valuation Allowance
| | |
2023 | | |
2022 | |
| | |
Year
Ended December 31, | |
| | |
2023 | | |
2022 | |
| Deferred Tax Assets | |
| | | |
| | |
| Net operating loss carryforwards | |
$ | 3,173,840 | | |
$ | 2,491,667 | |
| Research and development tax credits | |
| 6,635 | | |
| 2,705 | |
| Property and equipment and operating lease liability | |
| 49,220 | | |
| 53,960 | |
| Valuation allowance | |
| (3,229,695 | ) | |
| (2,548,331 | ) |
| Net deferred tax assets | |
$ | - | | |
$ | - | |
|
| X |
- DefinitionTabular disclosure of the components of net deferred tax asset or liability recognized in an entity's statement of financial position, including the following: the total of all deferred tax liabilities, the total of all deferred tax assets, the total valuation allowance recognized for deferred tax assets. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_ScheduleOfDeferredTaxAssetsAndLiabilitiesTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Cash and Cash Equivalents (Tables)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Schedule of Cash and Cash Equivalents |
Cash
and cash equivalents consist of the following:
Schedule
of Cash and Cash Equivalents
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Balances with banks | |
$ | 116,107 | | |
$ | 31,943 | |
| Money market securities(Highly liquid investments) | |
| - | | |
| 1,091,935 | |
| Total Cash and Cash Equivalents | |
$ | 116,106 | | |
$ | 1,123,878 | |
|
Cash
and cash equivalents consist of the following:
Schedule
of Cash and Cash Equivalents
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Balances with banks | |
$ | 31,943 | | |
$ | 604,813 | |
| Money market securities (Highly liquid investments) | |
| 1,091,935 | | |
| - | |
| Total Cash and Cash Equivalents | |
$ | 1,123,878 | | |
$ | 604,813 | |
|
| X |
- DefinitionTabular disclosure of the components of cash and cash equivalents.
| Name: |
us-gaap_ScheduleOfCashAndCashEquivalentsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Long-Term Convertible Debt, net of debt discount (Tables) - Scienture Inc [Member]
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Schedule of Long Term Convertible Debt Net of Discount |
Long-term
convertible debt, net of debt discount, consisted of the following:
Schedule of Long Term Convertible
Debt Net of Discount
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Principal | |
$ | 2,000,000 | | |
$ | 2,000,000 | |
| Less: Unamortized debt discount | |
| 265,339 | | |
| 374,883 | |
| Long-term convertible notes, net of debt discount | |
$ | 1,734,661 | | |
$ | 1,625,117 | |
|
Long-term
convertible debt, net of debt discount, consisted of the following:
Schedule of Long Term Convertible
Debt Net of Discount
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Principal | |
$ | 2,000,000 | | |
$ | - | |
| Less: Unamortized debt discount | |
| 374,883 | | |
| - | |
| Long-term convertible debt, net of debt discount | |
$ | 1,625,117 | | |
$ | - | |
|
| Schedule of Maturities Notes |
Maturities
of the outstanding notes are as follows:
Schedule of Maturities Notes
| Years Ending December 31 | |
| |
| 2024 | |
$ | - | |
| Year 1 | |
$ | - | |
| 2025 | |
| 2,000,000 | |
| Year 3 | |
| | |
| Total | |
$ | 2,000,000 | |
|
Maturities
of the outstanding debt are as follows:
Schedule of Maturities Notes
| Years Ending December 31 | |
| |
| 2024 | |
$ | - | |
| Year 2 | |
$ | - | |
| 2025 | |
| 2,000,000 | |
| Year 3 | |
| 2,000,000 | |
| Total | |
$ | 2,000,000 | |
|
| X |
- DefinitionTabular disclosure of contractual obligation by timing of payment due. Includes, but is not limited to, long-term debt obligation, lease obligation, and purchase obligation. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Name Regulation S-X (SX)
-Number 210
-Section 12
-Subsection 04
-Subparagraph (a)
-Publisher SEC
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
| Name: |
srt_ContractualObligationFiscalYearMaturityScheduleTableTextBlock |
| Namespace Prefix: |
srt_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of long-debt instruments or arrangements, including identification, terms, features, collateral requirements and other information necessary to a fair presentation. These are debt arrangements that originally required repayment more than twelve months after issuance or greater than the normal operating cycle of the entity, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69E
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69E
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 5: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
Reference 7: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 942
-SubTopic 470
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477734/942-470-50-3
Reference 8: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-8
Reference 9: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-6
Reference 10: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-7
| Name: |
us-gaap_ScheduleOfDebtInstrumentsTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Related Party Transactions (Tables)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Schedule of Relatives or Related Parties to Directors Purchased Securities |
Schedule of Relatives or Related Parties to Directors Purchased Securities
|
Relatives
or related parties to directors purchased securities from the Company on the same terms as unrelated parties as set forth below:
Schedule of Relatives or Related
Parties to Directors Purchased Securities
| Name of the Related
Party | |
Nature of transaction | |
Transactions
during the year ended
December 31 | | |
Balances
as at
December 31 | |
| | |
| |
2023 | | |
2022 | | |
2022 | | |
2023 | |
| Ms. Pushpa Shankar | |
Issue of Preferred Stock | |
$ | - | | |
$ | - | | |
$ | 750,000 | | |
$ | 750,000 | |
| Ms. Pushpa Shankar | |
Issue of Convertible notes | |
| 400,000 | | |
| 150,000 | | |
| 550,000 | | |
| 400,000 | |
| Ms. Yogita Desai | |
Issue of Preferred Stock | |
| - | | |
| - | | |
| 500,000 | | |
| 500,000 | |
| Ms. Yogita Desai | |
Issue of Convertible notes | |
| - | | |
| - | | |
| 100,000 | | |
| 100,000 | |
| Mr. Sandeep Gupta | |
Issue of Convertible notes | |
| - | | |
| 50,000 | | |
| 50,000 | | |
| - | |
| Total | |
| |
$ | 400,000 | | |
$ | 200,000 | | |
$ | 1,950,000 | | |
$ | 1,750,000 | |
|
| X |
- DefinitionTabular disclosure of related party transactions. Examples of related party transactions include, but are not limited to, transactions between (a) a parent company and its subsidiary; (b) subsidiaries of a common parent; (c) and entity and its principal owners and (d) affiliates.
| Name: |
us-gaap_ScheduleOfRelatedPartyTransactionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Scienture Inc. 2020 Stock Option and Grant Plan (Tables) - Scienture Inc [Member]
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Summary of Stock Option Activity |
A
summary of the Company’s stock option activity under the Plans is as follows:
Summary of Stock Option Activity
| | |
Outstanding
Options | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Remaining
Term
(Years) | |
| Balance as of December 31, 2023 | |
| 655,000 | | |
$ | 0.90 | | |
| 7.56 | |
| Granted | |
| 142,199 | | |
| 1.13 | | |
| | |
| Exercised | |
| - | | |
| - | | |
| | |
| Cancelled and forfeited | |
| - | | |
| - | | |
| | |
| Balance as of June 20, 2024 | |
| 797,199 | | |
$ | 0.94 | | |
| 6.21 | |
| | |
| | | |
| | | |
| | |
| Vested and exercisable as of June 30, 2024 | |
| 499,089 | | |
$ | 0.84 | | |
| 5.62 | |
|
A
summary of the Company’s stock option activity under the Plans is as follows:
Summary of Stock Option Activity
| | |
Outstanding
Options | | |
Weighted-
Average
Exercise
Price | | |
Weighted-
Average
Remaining
Term
(Years) | |
| Balance as of December 31, 2022 | |
| 560,000 | | |
$ | 0.83 | | |
| 7.95 | |
| Granted | |
| 185,000 | | |
| 1.13 | | |
| | |
| Exercised | |
| - | | |
| - | | |
| | |
| Cancelled and forfeited | |
| (90,000 | ) | |
| - | | |
| | |
| Balance as of December 31, 2023 | |
| 655,000 | | |
$ | 0.90 | | |
| 7.56 | |
| | |
| | | |
| | | |
| | |
| Vested and exercisable as of December 31, 2023 | |
| 410,065 | | |
$ | 0.84 | | |
| 6.84 | |
|
| Schedule of Stock-based Compensation Expense |
The
Company recorded stock-based compensation expense in the Statement of Operations and Comprehensive Loss for the periods presented as
follows:
Schedule of Stock-based Compensation Expense
| | |
2024 | | |
2023 | |
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| General and Administrative Expenses | |
$ | 44,837 | | |
$ | 39,724 | |
| Total stock-based compensation expense | |
$ | 44,837 | | |
$ | 39,724 | |
|
The
Company recorded stock-based compensation expense in the Statement of Operations and Comprehensive Loss for the periods presented as
follows:
Schedule of Stock-based Compensation Expense
| | |
2023 | | |
2022 | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| General and Administrative Expenses | |
$ | 79,449 | | |
$ | 68,087 | |
| Total stock-based compensation expense | |
$ | 79,449 | | |
$ | 68,087 | |
|
| Schedule of Stock Option Valuation Assumptions |
The
fair value of each employee option grant was estimated on the date of grant using the Black-Scholes option pricing model and the following
assumptions for the periods indicated:
Schedule of Stock Option Valuation Assumptions
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Expected volatility | |
| 72 | % | |
| 65%
- 75 | % |
| Risk-free interest rate | |
| 4.1%
- 4.4 | % | |
| 0.5%
- 3.5 | % |
| Expected term | |
| 5.7
- 6.1 years | | |
| 5.9
- 6.0 years | |
| Expected dividend | |
| 0 | % | |
| 0 | % |
|
The
fair value of each employee option grant was estimated on the date of grant using the Black-Scholes option pricing model and the following
assumptions for the periods indicated:
Schedule of Stock Option Valuation Assumptions
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Expected volatility | |
| 65%
- 75 | % | |
| 65%
- 75 | % |
| Risk-free interest rate | |
| 0.5%
- 3.5 | % | |
| 0.5%
- 2.8 | % |
| Expected term | |
| 5.9
- 6.0 years | | |
| 5.9
- 6.0 years | |
| Expected dividend | |
| 0 | % | |
| 0 | % |
|
| X |
- DefinitionTabular disclosure of share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_DisclosureOfShareBasedCompensationArrangementsByShareBasedPaymentAwardTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure for stock option plans. Includes, but is not limited to, outstanding awards at beginning and end of year, grants, exercises, forfeitures, and weighted-average grant date fair value. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)
-SubTopic 10
-Topic 718
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedCompensationStockOptionsActivityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of the significant assumptions used during the year to estimate the fair value of stock options, including, but not limited to: (a) expected term of share options and similar instruments, (b) expected volatility of the entity's shares, (c) expected dividends, (d) risk-free rate(s), and (e) discount for post-vesting restrictions. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (f)(2)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ScheduleOfShareBasedPaymentAwardStockOptionsValuationAssumptionsTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Warrants (Tables)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Scienture Inc [Member] |
|
|
| Summary of Assumptions Used to Estimate Fair Value of Warrants |
Summary of Assumptions Used to Estimate Fair Value of Warrants
|
The
following table summarizes the assumptions used to estimate the fair value of the outstanding warrants during the years ended December
31, 2023, and 2022:
Summary
of Assumptions Used to Estimate Fair Value of Warrants
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Expected dividend yield | |
| 0 | % | |
| - | |
| Weighted-average expected volatility | |
| 70.52 | % | |
| - | |
| Weighted-average risk-free interest rate | |
| 4.50 | % | |
| - | |
| Expected life of warrants | |
| 5
years | | |
| - | |
|
| X |
- DefinitionSummary of Assumptions Used to Estimate Fair Value of Warrants Granted [Table Text Block]
| Name: |
SCNX_SummaryOfAssumptionsUsedToEstimateFairValueOfWarrantsGrantedTableTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Leases (Tables) - Scienture Inc [Member]
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
| Schedule of Lease Expense |
The
components of lease expense were as follows:
Schedule
of Lease Expense
| | |
| | | |
| | |
| | |
June
30, | |
| | |
2024 | | |
2023 | |
| Operating lease costs | |
| | | |
| | |
| Amortization of ROU Assets | |
$ | 2,152 | | |
$ | - | |
| Interest on Lease Liabilities | |
$ | 1,190 | | |
$ | - | |
| Short term lease costs | |
$ | 17,010 | | |
$ | - | |
|
The
components of lease expense were as follows:
Schedule
of Lease Expense
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Operating lease costs | |
| | | |
| | |
| Amortization of ROU Assets | |
$ | 5,025 | | |
$ | - | |
| Interest on Lease Liabilities | |
$ | 2,380 | | |
$ | - | |
| Short term lease costs | |
$ | 34,021 | | |
$ | 32,677 | |
|
| Schedule of Supplemental Balance Sheet Information Related To Leases |
Supplemental
balance sheet information related to leases was as follows:
Schedule
of Supplemental Balance Sheet Information Related To Leases
| | |
June 30, | | |
December 31, | |
| | |
2024 | | |
2023 | |
| Operating Leases | |
| | | |
| | |
| Right of Use Assets | |
$ | 61,579 | | |
$ | 64,091 | |
| | |
| | | |
| | |
| Short term Lease liabilities | |
$ | 22,567 | | |
$ | 21,404 | |
| Long term Lease liabilities | |
$ | 39,319 | | |
$ | 42,893 | |
| Total Lease Liabilities | |
$ | 61,886 | | |
$ | 64,297 | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term (in years) | |
| 2.33 | | |
| 2.83 | |
| | |
| | | |
| | |
| Weighted Average Discount Rate | |
| 15.50 | % | |
| 15.50 | % |
|
Supplemental
balance sheet information related to leases was as follows:
Schedule
of Supplemental Balance Sheet Information Related To Leases
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Operating Leases | |
| | | |
| | |
| Right of Use Assets | |
$ | 64,091 | | |
$ | - | |
| | |
| | | |
| | |
| Short term Lease liabilities | |
$ | 21,404 | | |
$ | - | |
| Long term Lease liabilities | |
$ | 42,893 | | |
$ | - | |
| Total Lease Liabilities | |
$ | 64,297 | | |
$ | - | |
| | |
| | | |
| | |
| Weighted Average Remaining Lease Term (in years) | |
| 2.83 | | |
| - | |
| | |
| | | |
| | |
| Weighted Average Discount Rate | |
| 15.50 | % | |
| - | |
|
| Schedule of Supplemental Cash Flow Information Related To Leases |
|
Supplemental
cash flow information related to leases was as follows:
Schedule
of Supplemental Cash Flow Information Related To Leases
| | |
| | | |
| | |
| | |
December
31, | |
| | |
2023 | | |
2022 | |
| Cash paid for accounts included in the measurements of lease liabilities | |
| | |
| |
| Operating cash flows for Operating leases | |
$ | 7,200 | | |
$ | - | |
| Right of Use Assets obtained in exchange for new Lease
Liabilities | |
$ | 205 | | |
$ | - | |
|
| Schedule of Maturities Of Lease Liabilities |
|
Maturities
of lease liabilities were as follows at December 31, 2023:
Schedule
of Maturities Of Lease Liabilities
| December 31, 2023 | |
| |
| 2024 | |
$ | 29,017 | |
| 2025 | |
| 29,887 | |
| 2026 | |
| 17,823 | |
| Total lease payments | |
| 76,727 | |
| Less: Imputed interest | |
| 12,430 | |
| Total | |
$ | 64,297 | |
|
| X |
- DefinitionSchedule of Supplemental Balance Sheet Information Related to Leases [Table Text Block]
| Name: |
SCNX_ScheduleOfSupplementalBalanceSheetInformationRelatedToLeasesTableTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionSchedule of Supplemental Cash Flow Information Related to Leases [Table Text Block]
| Name: |
SCNX_ScheduleOfSupplementalCashFlowInformationRelatedToLeasesTableTextBlock |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of lessee's lease cost. Includes, but is not limited to, interest expense for finance lease, amortization of right-of-use asset for finance lease, operating lease cost, short-term lease cost, variable lease cost and sublease income. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_LeaseCostTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTabular disclosure of undiscounted cash flows of lessee's operating lease liability. Includes, but is not limited to, reconciliation of undiscounted cash flows to operating lease liability recognized in statement of financial position. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityMaturityTableTextBlock |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:textBlockItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Summary of Significant Accounting Policies (Details Narrative) - Scienture Inc [Member]
|
1 Months Ended |
6 Months Ended |
12 Months Ended |
|
Mar. 31, 2024
USD ($)
|
Jun. 30, 2024
USD ($)
Integer
|
Jun. 30, 2023
USD ($)
|
Dec. 31, 2023
USD ($)
Integer
|
Dec. 31, 2022
USD ($)
|
| Number of reportable segment | Integer |
|
1
|
|
1
|
|
| Number of operating segment | Integer |
|
1
|
|
1
|
|
| Allowance for doubtful accounts |
|
$ 0
|
|
$ 0
|
$ 0
|
| Revenue recognized |
|
|
$ 800,000
|
$ 800,000
|
$ 300,000
|
| Termination fee |
$ 1,285,000
|
1,285,000
|
|
|
|
| Termination fee description |
This
agreement also requires that if the full $1,285,00 has not been repaid within two years of the early of i) commercial launch or ii) 120
from FDA approval, then interest will accrue prospectively at a rate of 8% annually on unpaid balance. Accordingly, the Company recorded
a $1,285,000 termination fee liability. As of the date of issue of financial statements, the entire amount is outstanding.
|
|
|
|
|
| State income tax rate |
|
|
|
8.70%
|
7.25%
|
| Loss before tax |
|
$ 4,435,814
|
$ 471,570
|
$ 2,240,989
|
$ 3,708,378
|
| X |
- DefinitionTermination fee description.
| Name: |
SCNX_TerminationFeeDescription |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of allowance for credit loss on accounts receivable, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 326
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479344/326-20-45-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481962/310-10-50-4
| Name: |
us-gaap_AllowanceForDoubtfulAccountsReceivableCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of operating segments. An operating segment is a component of an enterprise: (a) that engages in business activities from which it may earn revenues and incur expenses (including revenues and expenses relating to transactions with other components of the same enterprise), (b) whose operating results are regularly reviewed by the enterprise's chief operating decision maker to make decisions about resources to be allocated to the segment and assess its performance, and (c) for which discrete financial information is available. An operating segment may engage in business activities for which it has yet to earn revenues, for example, start-up operations may be operating segments before earning revenues. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfOperatingSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of segments reported by the entity. A reportable segment is a component of an entity for which there is an accounting requirement to report separate financial information on that component in the entity's financial statements. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 47
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-47
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 54
-Subparagraph (e)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-54
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 18
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-18
| Name: |
us-gaap_NumberOfReportableSegments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:integerItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, excluding tax collected from customer, of revenue from satisfaction of performance obligation by transferring promised good or service to customer. Tax collected from customer is tax assessed by governmental authority that is both imposed on and concurrent with specific revenue-producing transaction, including, but not limited to, sales, use, value added and excise. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 41
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-41
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 4: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 5: http://fasb.org/us-gaap/role/ref/otherTransitionRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 924
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SAB Topic 11.L)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479941/924-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 5
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-5
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 30
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-30
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 42
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-42
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 40
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-40
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 606
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479806/606-10-50-4
| Name: |
us-gaap_RevenueFromContractWithCustomerExcludingAssessedTax |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Going Concern (Details Narrative) - Scienture Inc [Member] - USD ($)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Consolidation, Less than Wholly Owned Subsidiary, Parent Ownership Interest, Effects of Changes, Net [Line Items] |
|
|
|
|
| Net loss |
$ 4,435,814
|
$ 471,570
|
$ 2,240,989
|
$ 3,708,378
|
| Stockholders deficit |
$ 4,988,676
|
|
$ 4,539,152
|
$ 2,821,872
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_ConsolidationLessThanWhollyOwnedSubsidiaryParentOwnershipInterestEffectsOfChangesNetLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe portion of profit or loss for the period, net of income taxes, which is attributable to the parent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-6
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 9
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-9
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 805
-SubTopic 60
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (g)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147476176/805-60-65-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 323
-Name Accounting Standards Codification
-Section 65
-Paragraph 2
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478666/740-323-65-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(20))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482765/220-10-50-6
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-3
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (b)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-1
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 815
-SubTopic 40
-Name Accounting Standards Codification
-Section 65
-Paragraph 1
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480175/815-40-65-1
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-8
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 11
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-11
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 250
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483443/250-10-50-4
Reference 17: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-10
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section 45
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479105/946-220-45-7
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-04(18))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477250/944-220-S99-1
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-07(9))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-1
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(1)(d))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 23: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1A
-Subparagraph (SX 210.13-01(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1A
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(i))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(A))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 29: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iii)(B))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 30: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(4)(iv))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 31: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1B
-Subparagraph (SX 210.13-02(a)(5))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480097/470-10-S99-1B
Reference 32: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 60B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482689/260-10-45-60B
Reference 33: http://www.xbrl.org/2003/role/disclosureRef
-Topic 205
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483499/205-20-50-7
Reference 34: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 35: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1A
Reference 36: http://www.xbrl.org/2003/role/disclosureRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482790/220-10-45-1B
Reference 37: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-04(22))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478524/942-220-S99-1
| Name: |
us-gaap_NetIncomeLoss |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of equity (deficit) attributable to parent. Excludes temporary equity and equity attributable to noncontrolling interest. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(19))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.6-05(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-2
Reference 8: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(4)(b))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(6))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 220
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.6-09(7))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479134/946-220-S99-3
Reference 11: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.4-08(g)(1)(ii))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-1
Reference 12: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 323
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481687/323-10-50-3
Reference 13: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 825
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 28
-Subparagraph (f)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482907/825-10-50-28
Reference 14: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 310
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SAB Topic 4.E)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480418/310-10-S99-2
| Name: |
us-gaap_StockholdersEquity |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Cash and Cash Equivalents (Details) - Scienture Inc [Member] - USD ($)
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Balances with banks |
$ 116,107
|
$ 31,943
|
$ 604,813
|
| Money market securities (Highly liquid investments) |
|
1,091,935
|
|
| Total Cash and Cash Equivalents |
$ 116,106
|
$ 1,123,878
|
$ 604,813
|
| X |
- DefinitionAmount of currency on hand as well as demand deposits with banks or financial institutions. Includes other kinds of accounts that have the general characteristics of demand deposits. Also includes short-term, highly liquid investments that are both readily convertible to known amounts of cash and so near their maturity that they present insignificant risk of changes in value because of changes in interest rates. Excludes cash and cash equivalents within disposal group and discontinued operation. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483467/210-10-45-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 230
-SubTopic 10
-Section 45
-Paragraph 4
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-4
| Name: |
us-gaap_CashAndCashEquivalentsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionFor banks and other depository institutions: Includes cash on hand (currency and coin), cash items in process of collection, noninterest bearing deposits due from other financial institutions (including corporate credit unions), and noninterest bearing balances with the Federal Reserve Banks, Federal Home Loan Banks and central banks. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
| Name: |
us-gaap_CashAndDueFromBanks |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionInvestment in short-term money-market instruments (such as commercial paper, banker's acceptances, repurchase agreements, government securities, certificates of deposit, and so forth) which are highly liquid (that is, readily convertible to known amounts of cash) and so near their maturity that they present an insignificant risk of changes in value because of changes in interest rates. Generally, only investments with original maturities of three months or less qualify as cash equivalents by definition. Original maturity means an original maturity to the entity holding the investment. For example, both a three-month US Treasury bill and a three-year Treasury note purchased three months from maturity qualify as cash equivalents. However, a Treasury note purchased three-years ago does not become a cash equivalent when its remaining maturity is three months.
| Name: |
us-gaap_MoneyMarketFundsAtCarryingValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Convertible Notes (Details Narrative) - Scienture Inc [Member] - USD ($)
|
1 Months Ended |
6 Months Ended |
12 Months Ended |
Mar. 31, 2024 |
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Convertible notes issued bear interest rate percentage |
|
8.00%
|
8.00%
|
2.00%
|
| Debt outstanding principal amount |
$ 3,665,220
|
|
$ 3,665,220
|
$ 2,950,000
|
| Maturity date |
|
December 2023
|
December
31,2023
|
|
| Interest expenses |
|
|
$ 152,423
|
$ 76,705
|
| Preferred Stock [Member] |
|
|
|
|
| Conversion of debt, amount |
$ 276,233
|
|
|
|
| Conversion of debt, shares |
965,567
|
|
|
|
| X |
- DefinitionFace (par) amount of debt instrument at time of issuance. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482900/835-30-50-1
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
| Name: |
us-gaap_DebtInstrumentFaceAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionContractual interest rate for funds borrowed, under the debt agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
| Name: |
us-gaap_DebtInstrumentInterestRateStatedPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionDescription of the maturity date of the debt instrument including whether the debt matures serially and, if so, a brief description of the serial maturities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_DebtInstrumentMaturityDateDescription |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest expense classified as operating and nonoperating. Includes, but is not limited to, cost of borrowing accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-24
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of shares issued during the period as a result of the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe gross value of stock issued during the period upon the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_StatementEquityComponentsAxis=us-gaap_PreferredStockMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Long Term Convertible Debt Net of Discount (Details) - Scienture Inc [Member] - USD ($)
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Principal |
$ 2,000,000
|
$ 2,000,000
|
|
| Less: Unamortized debt discount |
265,339
|
374,883
|
|
| Long-term convertible debt, net of debt discount |
$ 1,734,661
|
$ 1,625,117
|
|
| X |
- DefinitionAmount, before unamortized (discount) premium and debt issuance costs, of long-term debt. Includes, but is not limited to, notes payable, bonds payable, commercial loans, mortgage loans, convertible debt, subordinated debt and other types of debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-4
| Name: |
us-gaap_DebtInstrumentCarryingAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after accumulated amortization, of debt discount. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 1A
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-1A
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 45
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482925/835-30-45-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482900/835-30-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
| Name: |
us-gaap_DebtInstrumentUnamortizedDiscount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt. Excludes lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-4
| Name: |
us-gaap_LongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Maturities Notes (Details) - Scienture Inc [Member] - USD ($)
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Year 1 |
|
|
|
| Year 2 |
2,000,000
|
|
|
| Year 3 |
|
2,000,000
|
|
| Total |
$ 2,000,000
|
$ 2,000,000
|
|
| X |
- DefinitionAmount, before unamortized (discount) premium and debt issuance costs, of long-term debt. Includes, but is not limited to, notes payable, bonds payable, commercial loans, mortgage loans, convertible debt, subordinated debt and other types of debt. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-4
| Name: |
us-gaap_DebtInstrumentCarryingAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 470
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-1
| Name: |
us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-SubTopic 10
-Topic 470
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481544/470-10-50-1
| Name: |
us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalInYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of long-term debt payable, sinking fund requirement, and other securities issued that are redeemable by holder at fixed or determinable price and date, maturing in remainder of current fiscal year. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 235
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 3
-Subparagraph (SX 210.12-04(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480678/235-10-S99-3
| Name: |
us-gaap_LongTermDebtMaturitiesRepaymentsOfPrincipalRemainderOfFiscalYear |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Long-Term Convertible Debt, net of debt discount (Details Narrative) - Scienture Inc [Member] - USD ($)
|
6 Months Ended |
12 Months Ended |
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
Sep. 30, 2023 |
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Long term debt |
$ 1,734,661
|
$ 1,625,117
|
|
|
| Debt interest rate percentage |
8.00%
|
8.00%
|
2.00%
|
|
| Interest expense |
|
$ 152,423
|
$ 76,705
|
|
| Loan Agreement [Member] |
|
|
|
|
| Collaborative Arrangement and Arrangement Other than Collaborative [Line Items] |
|
|
|
|
| Long term debt |
|
|
|
$ 2,000,000
|
| Debt prime rate percentage |
|
7.00%
|
|
|
| Debt interest rate percentage |
15.50%
|
15.50%
|
|
|
| Interest expense |
|
$ 95,583
|
|
|
| Debt diluted valuation |
$ 60,000,000
|
$ 60,000,000
|
|
|
| Number of warrant to purchase |
509,014
|
509,014
|
|
|
| Fair value adjustment of warrants |
$ 444,260
|
$ 444,260
|
|
|
| Debt discount interest expense |
$ 109,544
|
$ 69,377
|
|
|
| X |
- DefinitionDebt instrument interest rate stated prime rate percentage.
| Name: |
SCNX_DebtInstrumentInterestRateStatedPrimeRatePercentage |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe aggregate amount of recurring noncash expense charged against earnings in the period to allocate the cost of assets over their estimated remaining economic lives. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_AdjustmentForAmortization |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of securities into which the class of warrant or right may be converted. For example, but not limited to, 500,000 warrants may be converted into 1,000,000 shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightNumberOfSecuritiesCalledByWarrantsOrRights |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 808
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479402/808-10-50-1
| Name: |
us-gaap_CollaborativeArrangementsAndNoncollaborativeArrangementTransactionsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionContractual interest rate for funds borrowed, under the debt agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
| Name: |
us-gaap_DebtInstrumentInterestRateStatedPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense (income) related to adjustment to fair value of warrant liability. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 25
-Paragraph 13
-SubTopic 10
-Topic 480
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481766/480-10-25-13
| Name: |
us-gaap_FairValueAdjustmentOfWarrants |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of interest expense classified as operating and nonoperating. Includes, but is not limited to, cost of borrowing accounted for as interest expense. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 49
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-49
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 270
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482964/270-10-50-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 32
-Subparagraph (ee)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-32
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 24
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-24
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 22
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482810/280-10-50-22
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 835
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483013/835-20-50-1
| Name: |
us-gaap_InterestExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount, after deduction of unamortized premium (discount) and debt issuance cost, of long-term debt. Excludes lease obligation. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22))
-SubTopic 10
-Topic 210
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/exampleRef
-Topic 835
-SubTopic 30
-Name Accounting Standards Codification
-Section 55
-Paragraph 8
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482949/835-30-55-8
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(16))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 4: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69B
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69B
Reference 5: http://www.xbrl.org/2003/role/exampleRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 69C
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481568/470-20-55-69C
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1D
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1D
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (b)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-4
| Name: |
us-gaap_LongTermDebt |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_TypeOfArrangementAxis=SCNX_LoanAgreementMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Related Party Transactions (Details Narrative) - Scienture Inc [Member] - USD ($)
|
1 Months Ended |
6 Months Ended |
12 Months Ended |
|
Mar. 31, 2024 |
Jun. 30, 2024 |
Jun. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
Jul. 31, 2024 |
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
| Termination fee |
$ 1,285,000
|
$ 1,285,000
|
|
|
|
|
| Lease payments |
|
14,440
|
0
|
$ 7,200
|
$ 0
|
|
| Research and Development Expenses |
|
1,520,947
|
946,435
|
2,029,812
|
3,061,493
|
|
| Subsequent Event [Member] |
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
| Convertible note |
$ 3,665,220
|
|
|
|
|
|
| Short term |
|
|
|
|
|
$ 250,000
|
| Promissory Note [Member] | Subsequent Event [Member] |
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
| Short term |
|
|
|
|
|
$ 265,000
|
| Kesin Pharma Corporation [Member] |
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
| Milestone structure |
|
0
|
800,000
|
800,000
|
300,000
|
|
| Saptalis Pharmaceuticals LLC [Member] |
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
| Research and Development Expenses |
|
$ 106,539
|
189,027
|
$ 355,124
|
$ 647,566
|
|
| Director [Member] |
|
|
|
|
|
|
| Related Party Transaction [Line Items] |
|
|
|
|
|
|
| Convertible note |
|
|
$ 250,000
|
|
|
|
| X |
- DefinitionAmount of expense for research and development. Includes, but is not limited to, cost for computer software product to be sold, leased, or otherwise marketed and writeoff of research and development assets acquired in transaction other than business combination or joint venture formation or both. Excludes write-down of intangible asset acquired in business combination or from joint venture formation or both, used in research and development activity. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 280
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 48
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482785/280-10-55-48
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 985
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481283/985-20-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 730
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482916/730-10-50-1
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 912
-SubTopic 730
-Name Accounting Standards Codification
-Section 25
-Paragraph 1
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479532/912-730-25-1
| Name: |
us-gaap_ResearchAndDevelopmentExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionReflects the total carrying amount as of the balance sheet date of debt having initial terms less than one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_ShortTermBorrowings |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionThe gross value of stock issued during the period upon the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow for variable lease payment excluded from lease liability. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (d)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-5
| Name: |
us-gaap_VariableLeasePayment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_DebtInstrumentAxis=SCNX_PromissoryNoteMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Summary of Stock Option Activity (Details) - Share-Based Payment Arrangement, Option [Member] - Scienture Inc [Member] - $ / shares
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Share-Based Compensation Arrangement by Share-Based Payment Award [Line Items] |
|
|
|
| Outstanding Options, Balance |
655,000
|
560,000
|
|
| Weighted- Average Exercise Price, Balance |
$ 0.90
|
$ 0.83
|
|
| Weighted-Average Remaining Term (Years), Balance |
6 years 2 months 15 days
|
7 years 6 months 21 days
|
7 years 11 months 12 days
|
| Outstanding Options, Granted |
142,199
|
185,000
|
|
| Weighted- Average Exercise Price, Granted |
$ 1.13
|
$ 1.13
|
|
| Outstanding Options, Exercised |
|
|
|
| Weighted- Average Exercise Price, Exercised |
|
|
|
| Outstanding Options, Cancelled and forfeited |
|
(90,000)
|
|
| Weighted- Average Exercise Price, Cancelled and forfeited |
|
|
|
| Outstanding Options, Balance |
797,199
|
655,000
|
560,000
|
| Weighted- Average Exercise Price, Balance |
$ 0.94
|
$ 0.90
|
$ 0.83
|
| Outstanding Options, Vested and exercisable |
499,089
|
410,065
|
|
| Outstanding Options, Vested and exercisable |
$ 0.84
|
$ 0.84
|
|
| Weighted-Average Remaining Term (Years), Vested and exercisable |
5 years 7 months 13 days
|
6 years 10 months 2 days
|
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe number of shares into which fully or partially vested stock options outstanding as of the balance sheet date can be currently converted under the option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe weighted-average price as of the balance sheet date at which grantees can acquire the shares reserved for issuance on vested portions of options outstanding and currently exercisable under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsExercisableWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionThe number of shares under options that were cancelled during the reporting period as a result of occurrence of a terminating event specified in contractual agreements pertaining to the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsForfeituresInPeriod |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionGross number of share options (or share units) granted during the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsGrantsInPeriodGross |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of options outstanding, including both vested and non-vested options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingNumber |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which grantees can acquire the shares reserved for issuance under the stock option plan. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardOptionsOutstandingWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average price at which option holders acquired shares when converting their stock options into shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsExercisesInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average price at which grantees could have acquired the underlying shares with respect to stock options that were terminated. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsForfeituresInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average per share amount at which grantees can acquire shares of common stock by exercise of options. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementsByShareBasedPaymentAwardOptionsGrantsInPeriodWeightedAverageExercisePrice |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for vested portions of options outstanding and currently exercisable or convertible, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 718
-SubTopic 10
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsExercisableWeightedAverageRemainingContractualTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average remaining contractual term for option awards outstanding, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents the reported fact of one year, five months, and thirteen days. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 718
-SubTopic 10
-Subparagraph (e)(1)
-Name Accounting Standards Codification
-Paragraph 2
-Section 50
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsOutstandingWeightedAverageRemainingContractualTerm2 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionNumber of share options (or share units) exercised during the current period. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(28))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodSharesStockOptionsExercised |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Stock-based Compensation Expense (Details) - Scienture Inc [Member] - USD ($)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Share-Based Payment Arrangement, Expensed and Capitalized, Amount [Line Items] |
|
|
|
|
| Total stock-based compensation expense |
$ 44,837
|
$ 39,724
|
$ 79,449
|
$ 68,087
|
| General and Administrative Expense [Member] |
|
|
|
|
| Share-Based Payment Arrangement, Expensed and Capitalized, Amount [Line Items] |
|
|
|
|
| Total stock-based compensation expense |
$ 44,837
|
$ 39,724
|
$ 79,449
|
$ 68,087
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_EmployeeServiceShareBasedCompensationAllocationOfRecognizedPeriodCostsLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of noncash expense for share-based payment arrangement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (a)
-SubTopic 10
-Topic 230
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_ShareBasedCompensation |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_IncomeStatementLocationAxis=us-gaap_GeneralAndAdministrativeExpenseMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
| X |
- DefinitionThe estimated dividend rate (a percentage of the share price) to be paid (expected dividends) to holders of the underlying shares over the option's term. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedDividendRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe estimated measure of the percentage by which a share price is expected to fluctuate during a period. Volatility also may be defined as a probability-weighted measure of the dispersion of returns about the mean. The volatility of a share price is the standard deviation of the continuously compounded rates of return on the share over a specified period. That is the same as the standard deviation of the differences in the natural logarithms of the stock prices plus dividends, if any, over the period. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsExpectedVolatilityRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe risk-free interest rate assumption that is used in valuing an option on its own shares. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardFairValueAssumptionsRiskFreeInterestRate |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionExpected term of award under share-based payment arrangement, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardFairValueAssumptionsExpectedTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MinimumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_RangeAxis=srt_MaximumMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
| X |
- DefinitionStock option and grant plan have contractual term.
| Name: |
SCNX_StockOptionAndGrantPlanHaveContractualTerm |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 1D
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-1D
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 35
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480483/718-10-35-3
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (a)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 6: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 7: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 8: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 9: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 10: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 11: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 12: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 13: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(1)(iv)(04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 14: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 15: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 16: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 17: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(01)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 18: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(02)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 19: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)(2)(iii)(03)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 20: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 21: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (d)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 22: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 23: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (e)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 24: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 25: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(ii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 26: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iii)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 27: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(iv)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
Reference 28: http://www.xbrl.org/2003/role/disclosureRef
-Topic 718
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (f)(2)(v)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480429/718-10-50-2
| Name: |
us-gaap_ShareBasedCompensationArrangementByShareBasedPaymentAwardLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionWeighted average grant-date fair value of options vested.
| Name: |
us-gaap_SharebasedCompensationArrangementBySharebasedPaymentAwardOptionsVestedWeightedAverageGrantDateFairValue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AwardTypeAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
| X |
- DefinitionExercise price per share or per unit of warrants or rights outstanding. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_ClassOfWarrantOrRightExercisePriceOfWarrantsOrRights1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:perShareItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of warrants or rights outstanding.
| Name: |
us-gaap_ClassOfWarrantOrRightOutstanding |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Net Loss per Share (Details Narrative) - Scienture Inc [Member] - shares
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Share-Based Payment Arrangement, Option [Member] |
|
|
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
|
|
| Antidilutive securities excluded from computation of earnings per share, amount |
799,199
|
655,000
|
655,000
|
560,000
|
| Warrant [Member] |
|
|
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
|
|
| Antidilutive securities excluded from computation of earnings per share, amount |
509,014
|
0
|
509,014
|
0
|
| Convertible Preferred Stock and Convertible Notes [Member] |
|
|
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
|
|
| Antidilutive securities excluded from computation of earnings per share, amount |
3,365,669
|
3,195,911
|
3,365,669
|
3,195,911
|
| Long Term Convertible Debt [Member] |
|
|
|
|
| Antidilutive Securities Excluded from Computation of Earnings Per Share [Line Items] |
|
|
|
|
| Antidilutive securities excluded from computation of earnings per share, amount |
0
|
3,350,000
|
9,529,683
|
0
|
| X |
- DefinitionSecurities (including those issuable pursuant to contingent stock agreements) that could potentially dilute basic earnings per share (EPS) or earnings per unit (EPU) in the future that were not included in the computation of diluted EPS or EPU because to do so would increase EPS or EPU amounts or decrease loss per share or unit amounts for the period presented. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 260
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 1
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482662/260-10-50-1
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_EmployeeStockOptionMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=us-gaap_WarrantMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=SCNX_ConvertiblePreferredStockAndConvertibleNotesMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_AntidilutiveSecuritiesExcludedFromComputationOfEarningsPerShareByAntidilutiveSecuritiesAxis=SCNX_LongTermConvertibleDebtMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Lease Expense (Details) - Scienture Inc [Member] - USD ($)
|
6 Months Ended |
12 Months Ended |
Jun. 30, 2024 |
Jun. 30, 2023 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Amortization of ROU Assets |
$ 2,152
|
|
$ 5,025
|
|
| Interest on Lease Liabilities |
1,190
|
|
2,380
|
|
| Short term lease costs |
$ 17,010
|
|
$ 34,021
|
$ 32,677
|
| X |
- DefinitionOperating lease interest expense.
| Name: |
SCNX_OperatingLeaseInterestExpense |
| Namespace Prefix: |
SCNX_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of periodic reduction over lease term of carrying amount of right-of-use asset from operating lease. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 230
-SubTopic 10
-Name Accounting Standards Codification
-Section 45
-Paragraph 28
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482740/230-10-45-28
| Name: |
us-gaap_OperatingLeaseRightOfUseAssetAmortizationExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of short-term lease cost, excluding expense for lease with term of one month or less. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_ShortTermLeaseCost |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Supplemental Balance Sheet Information Related To Leases (Details) - Scienture Inc [Member] - USD ($)
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Right of Use Assets |
$ 61,579
|
$ 64,091
|
|
| Short term Lease liabilities |
22,567
|
21,404
|
|
| Long term Lease liabilities |
39,319
|
42,893
|
|
| Total Lease Liabilities |
$ 61,886
|
$ 64,297
|
|
| Weighted Average Remaining Lease Term (in years) |
2 years 3 months 29 days
|
2 years 9 months 29 days
|
|
| Weighted Average Discount Rate |
15.50%
|
15.50%
|
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as current. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityCurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease, classified as noncurrent. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiabilityNoncurrent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's right to use underlying asset under operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseRightOfUseAsset |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average discount rate for operating lease calculated at point in time. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(4)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageDiscountRatePercent |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionWeighted average remaining lease term for operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(3)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeaseWeightedAverageRemainingLeaseTerm1 |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
| X |
- DefinitionRemaining lease term of operating lease, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 7A
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-7A
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 3
-Subparagraph (a)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-3
| Name: |
us-gaap_LesseeOperatingLeaseRemainingLeaseTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Deferred Tax Assets and Related Valuation Allowance (Details) - Scienture Inc [Member] - USD ($)
|
Dec. 31, 2023 |
Dec. 31, 2022 |
| Net operating loss carryforwards |
$ 3,173,840
|
$ 2,491,667
|
| Research and development tax credits |
6,635
|
2,705
|
| Property and equipment and operating lease liability |
49,220
|
53,960
|
| Valuation allowance |
(3,229,695)
|
(2,548,331)
|
| Net deferred tax assets |
|
|
| X |
- DefinitionAmount after allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences and carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsNet |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible operating loss carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsOperatingLossCarryforwards |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible temporary differences from property, plant, and equipment. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsPropertyPlantAndEquipment |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount before allocation of valuation allowances of deferred tax asset attributable to deductible research tax credit carryforwards. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-6
| Name: |
us-gaap_DeferredTaxAssetsTaxCreditCarryforwardsResearch |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of deferred tax assets for which it is more likely than not that a tax benefit will not be realized. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 740
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482685/740-10-50-2
| Name: |
us-gaap_DeferredTaxAssetsValuationAllowance |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Relatives or Related Parties to Directors Purchased Securities (Details) - Scienture Inc [Member] - USD ($)
|
12 Months Ended |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Defined Benefit Plan Disclosure [Line Items] |
|
|
| Related party Transactions |
$ 400,000
|
$ 200,000
|
| Related party Balances |
$ 1,750,000
|
1,950,000
|
| Ms. Pushpa Shankar [Member] | Issue of Preferred Stock [Member] |
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
| Nature of transaction |
Issue of Preferred Stock
|
|
| Related party Transactions |
|
|
| Related party Balances |
$ 750,000
|
750,000
|
| Ms. Pushpa Shankar [Member] | Issue of Convertible Notes [Member] |
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
| Nature of transaction |
Issue of Convertible notes
|
|
| Related party Transactions |
$ 400,000
|
150,000
|
| Related party Balances |
$ 400,000
|
550,000
|
| Ms. Yogita Desai [Member] | Issue of Preferred Stock [Member] |
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
| Nature of transaction |
Issue of Preferred Stock
|
|
| Related party Transactions |
|
|
| Related party Balances |
$ 500,000
|
500,000
|
| Ms. Yogita Desai [Member] | Issue of Convertible Notes [Member] |
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
| Nature of transaction |
Issue of Convertible notes
|
|
| Related party Transactions |
|
|
| Related party Balances |
$ 100,000
|
100,000
|
| Mr. Sandeep Gupta [Member] | Issue of Convertible Notes [Member] |
|
|
| Defined Benefit Plan Disclosure [Line Items] |
|
|
| Nature of transaction |
Issue of Convertible notes
|
|
| Related party Transactions |
|
50,000
|
| Related party Balances |
|
$ 50,000
|
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table.
| Name: |
us-gaap_DefinedBenefitPlanDisclosureLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of liabilities classified as other. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(15))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 946
-SubTopic 830
-Name Accounting Standards Codification
-Section 55
-Paragraph 12
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479168/946-830-55-12
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(2))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 5: http://www.xbrl.org/2003/role/disclosureRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 6: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.6-04(12)(b)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479170/946-210-S99-1
Reference 7: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(17))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_OtherLiabilities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
| X |
- DefinitionLine items represent financial concepts included in a table. These concepts are used to disclose reportable information associated with domain members defined in one or many axes to the table. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 103
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482078/820-10-55-103
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_FairValueAssetsAndLiabilitiesMeasuredOnRecurringAndNonrecurringBasisValuationTechniquesLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionPeriod between issuance and expiration of outstanding warrant and right embodying unconditional obligation requiring redemption by transferring asset at specified or determinable date or upon event certain to occur, in 'PnYnMnDTnHnMnS' format, for example, 'P1Y5M13D' represents reported fact of one year, five months, and thirteen days. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)(i)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
Reference 3: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 820
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Subparagraph (bbb)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147482106/820-10-50-2
| Name: |
us-gaap_WarrantsAndRightsOutstandingTerm |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:durationItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
| X |
- References
+ Details
| Name: |
us-gaap_CashFlowNoncashInvestingAndFinancingActivitiesDisclosureAbstract |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of cash outflow from operating lease, excluding payments to bring another asset to condition and location necessary for its intended use. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 5
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-5
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(1)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_OperatingLeasePayments |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionAmount of increase in right-of-use asset obtained in exchange for operating lease liability. Reference 1: http://www.xbrl.org/2003/role/exampleRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 55
-Paragraph 53
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479589/842-20-55-53
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 4
-Subparagraph (g)(2)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-4
| Name: |
us-gaap_RightOfUseAssetObtainedInExchangeForOperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Schedule of Maturities Of Lease Liabilities (Details) - Scienture Inc [Member] - USD ($)
|
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| 2024 |
|
$ 29,017
|
|
| 2025 |
|
29,887
|
|
| 2026 |
|
17,823
|
|
| Total lease payments |
|
76,727
|
|
| Less: Imputed interest |
|
12,430
|
|
| Total |
$ 61,886
|
$ 64,297
|
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDue |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in next fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueNextTwelveMonths |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in third fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearThree |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payment for operating lease to be paid in second fiscal year following current fiscal year. Excludes interim and annual periods when interim periods are reported from current statement of financial position date (rolling approach). Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityPaymentsDueYearTwo |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of lessee's undiscounted obligation for lease payments in excess of discounted obligation for lease payments for operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 6
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478964/842-20-50-6
| Name: |
us-gaap_LesseeOperatingLeaseLiabilityUndiscountedExcessAmount |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionPresent value of lessee's discounted obligation for lease payments from operating lease. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 842
-SubTopic 20
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147479041/842-20-45-1
| Name: |
us-gaap_OperatingLeaseLiability |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
v3.24.4
Subsequent Events (Details Narrative) - Scienture Inc [Member] - USD ($)
|
1 Months Ended |
|
|
|
|
Mar. 31, 2024 |
Jul. 31, 2024 |
Jun. 30, 2024 |
Dec. 31, 2023 |
Dec. 31, 2022 |
| Subsequent Event [Line Items] |
|
|
|
|
|
| Debt instrument interest rate stated percentage |
|
|
8.00%
|
8.00%
|
2.00%
|
| Subsequent Event [Member] |
|
|
|
|
|
| Subsequent Event [Line Items] |
|
|
|
|
|
| Short term borrowings |
|
$ 250,000
|
|
|
|
| Stock issued during period value conversion of convertible securities |
$ 3,665,220
|
|
|
|
|
| Interest receivable |
$ 276,233
|
|
|
|
|
| Stock issued during period shares conversion of convertible securities |
965,568
|
|
|
|
|
| Royalty expense |
$ 1,285,000
|
|
|
|
|
| Debt instrument interest rate stated percentage |
8.00%
|
|
|
|
|
| X |
- DefinitionContractual interest rate for funds borrowed, under the debt agreement. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(22)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1B
-Subparagraph (b)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1B
| Name: |
us-gaap_DebtInstrumentInterestRateStatedPercentage |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
dtr-types:percentItemType |
| Balance Type: |
na |
| Period Type: |
instant |
|
| X |
- DefinitionCarrying amount as of the balance sheet date of interest earned but not received. Also called accrued interest or accrued interest receivable. Reference 1: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 946
-SubTopic 310
-Name Accounting Standards Codification
-Section 45
-Paragraph 1
-Subparagraph (a)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147477802/946-310-45-1
Reference 2: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(3)(a)(4))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
| Name: |
us-gaap_InterestReceivable |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
instant |
|
| X |
- DefinitionAmount of expense related to royalty payments under a contractual arrangement such as payment for mineral and drilling rights and use of technology or intellectual property. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 220
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 2
-Subparagraph (SX 210.5-03(3))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483621/220-10-S99-2
| Name: |
us-gaap_RoyaltyExpense |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
debit |
| Period Type: |
duration |
|
| X |
- DefinitionReflects the total carrying amount as of the balance sheet date of debt having initial terms less than one year or the normal operating cycle, if longer. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(19)(a))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 942
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.9-03(13))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478546/942-210-S99-1
Reference 3: http://www.xbrl.org/2003/role/exampleRef
-Topic 852
-SubTopic 10
-Name Accounting Standards Codification
-Section 55
-Paragraph 10
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481372/852-10-55-10
Reference 4: http://www.xbrl.org/2009/role/commonPracticeRef
-Topic 944
-SubTopic 210
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.7-03(a)(16)(a)(1))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147478777/944-210-S99-1
| Name: |
us-gaap_ShortTermBorrowings |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
instant |
|
| X |
- DefinitionNumber of shares issued during the period as a result of the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://www.xbrl.org/2003/role/disclosureRef
-Topic 470
-SubTopic 20
-Name Accounting Standards Codification
-Section 50
-Paragraph 1E
-Subparagraph (c)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481139/470-20-50-1E
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
Reference 6: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Topic 505
-SubTopic 10
-Section 50
-Paragraph 3
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-3
| Name: |
us-gaap_StockIssuedDuringPeriodSharesConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:sharesItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe gross value of stock issued during the period upon the conversion of convertible securities. Reference 1: http://fasb.org/us-gaap/role/ref/legacyRef
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-SubTopic 10
-Topic 505
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481112/505-10-50-2
Reference 2: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(29))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 3: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(30))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 4: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 210
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.5-02(31))
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480566/210-10-S99-1
Reference 5: http://fasb.org/us-gaap/role/ref/legacyRef
-Topic 505
-SubTopic 10
-Name Accounting Standards Codification
-Section S99
-Paragraph 1
-Subparagraph (SX 210.3-04)
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147480008/505-10-S99-1
| Name: |
us-gaap_StockIssuedDuringPeriodValueConversionOfConvertibleSecurities |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:monetaryItemType |
| Balance Type: |
credit |
| Period Type: |
duration |
|
| X |
- DefinitionDetail information of subsequent event by type. User is expected to use existing line items from elsewhere in the taxonomy as the primary line items for this disclosure, which is further associated with dimension and member elements pertaining to a subsequent event. Reference 1: http://www.xbrl.org/2003/role/disclosureRef
-Topic 830
-SubTopic 30
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147481674/830-30-50-2
Reference 2: http://www.xbrl.org/2003/role/disclosureRef
-Topic 855
-SubTopic 10
-Name Accounting Standards Codification
-Section 50
-Paragraph 2
-Publisher FASB
-URI https://asc.fasb.org/1943274/2147483399/855-10-50-2
| Name: |
us-gaap_SubsequentEventLineItems |
| Namespace Prefix: |
us-gaap_ |
| Data Type: |
xbrli:stringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Details
| Name: |
srt_ConsolidatedEntitiesAxis=SCNX_ScientureIncMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
| X |
- Details
| Name: |
us-gaap_SubsequentEventTypeAxis=us-gaap_SubsequentEventMember |
| Namespace Prefix: |
|
| Data Type: |
na |
| Balance Type: |
|
| Period Type: |
|
|
Scienture (NASDAQ:SCNX)
Graphique Historique de l'Action
De Déc 2024 à Jan 2025

Scienture (NASDAQ:SCNX)
Graphique Historique de l'Action
De Jan 2024 à Jan 2025
