Profound Medical to Release Second Quarter 2024 Financial Results on August 8 – Conference Call to Follow
18 Juillet 2024 - 10:30PM

Profound Medical Corp. (NASDAQ:PROF; TSX:PRN) (“Profound” or the
“Company”), a commercial-stage medical device company that develops
and markets customizable, incision-free therapies for the ablation
of diseased tissue, will announce its second quarter 2024 financial
results after market close on Thursday, August 8, 2024.
Profound management will host a conference call
at 4:30 p.m. ET to review the financial results and discuss
business developments in the period.
Second Quarter 2024 Results Conference
Call Details:
|
Date: |
|
Thursday, August 8, 2024 |
|
|
|
|
|
Time: |
|
4:30 p.m. ET |
|
|
|
|
|
Live Call Registration: |
|
https://register.vevent.com/register/BIbfa8c9bee6c6463c8ca3a1937fbfd804 |
|
|
|
|
The call will also be broadcast live and
archived on the Company's website at www.profoundmedical.com under
"Webcasts" in the Investors section.
About Profound Medical
Corp.
Profound is a commercial-stage medical device
company that develops and markets customizable, incision-free
therapies for the ablation of diseased tissue.
Profound is commercializing TULSA-PRO®, a
technology that combines real-time MRI, robotically-driven
transurethral ultrasound and closed-loop temperature feedback
control. TULSA-PRO® is designed to provide customizable and
predictable radiation-free ablation of a surgeon-defined prostate
volume while actively protecting the urethra and rectum to help
preserve the patient’s natural functional abilities.
TULSA-PRO® has the potential to be a flexible technology in
customizable prostate ablation, including intermediate stage
cancer, localized radio-recurrent cancer, retention and hematuria
palliation in locally advanced prostate cancer, and the transition
zone in large volume benign prostatic hyperplasia (“BPH”).
TULSA-PRO® is CE marked, Health Canada approved, and 510(k) cleared
by the U.S. Food and Drug Administration (“FDA”).
Profound is also commercializing Sonalleve®, an
innovative therapeutic platform that is CE marked for the treatment
of uterine fibroids and palliative pain treatment of bone
metastases. Sonalleve® has also been approved by the China
National Medical Products Administration for the non-invasive
treatment of uterine fibroids and has FDA approval under a
Humanitarian Device Exemption for the treatment of osteoid osteoma.
The Company is in the early stages of exploring additional
potential treatment markets for Sonalleve® where the
technology has been shown to have clinical application, such as
non-invasive ablation of abdominal cancers and hyperthermia for
cancer therapy.
For further information, please
contact:
Stephen KilmerInvestor
Relationsskilmer@profoundmedical.com T: 647.872.4849
Profound Medical (TSX:PRN)
Graphique Historique de l'Action
De Oct 2024 à Nov 2024
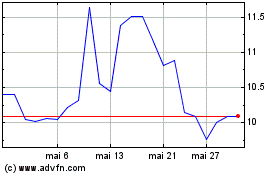
Profound Medical (TSX:PRN)
Graphique Historique de l'Action
De Nov 2023 à Nov 2024