Biotalys Receives Approval for Large-Scale Demonstration Trials of EVOCA in the Netherlands
02 Septembre 2024 - 7:00AM
UK Regulatory
Biotalys Receives Approval for Large-Scale Demonstration Trials of
EVOCA in the Netherlands
Ghent, Belgium, Sept. 02, 2024 (GLOBE NEWSWIRE) --
Press release
Biotalys (Euronext: BTLS) an Agricultural
Technology (AgTech) company developing protein-based biocontrols
for sustainable crop protection, today announced it received
approval by the Dutch regulator CTGB (College voor de Toelating van
Gewasbeschermingsmiddelen en Biociden) for large-scale
demonstration trials in greenhouses of its first biofungicide
candidate, EVOCA™*. Importantly, the harvested fruits and
vegetables can be sold for human consumption.
Jeannette Vriend, Plant protection
specialist at Dutch growers association Glastuinbouw Nederland,
said: “We are very pleased that this innovative technology
can now be tested at large scale by growers. Given the many
challenges to adequately control fungal diseases in tomatoes,
cucumbers and strawberries, due to a sharply shrinking crop
protection product package, we really need the acceleration of new,
green solutions. These trials offer an ideal opportunity to
properly implement such solutions in these high-value integrated
crops.”
The CTGB granted Biotalys the approval to test
EVOCA against powdery mildew in 40 hectares of tomatoes, 20
hectares of cucumbers and 10 hectares of strawberries. Produce from
these greenhouse trials is allowed to be sold for human
consumption, an exemption to standard practices requiring crop
destruction when a crop protection product is used that has not yet
received regulatory approval.**
Eva Van Hende, Head of Regulatory and
Sustainability of Biotalys, said: “The
ability to test EVOCA in large-scale demonstration trials while
allowing the sale of the harvested produce for consumption
reinforces our confidence that the product is safe to use. This
decision by the Dutch authority is particularly relevant as the
Netherlands is both the rapporteur Member State for our regulatory
dossier at the European level and one of the largest exporters of
fruits and vegetables worldwide.”
EVOCA is a novel protein-based biofungicide
which earned an entirely new resistance classification by the
Fungicide Resistance Action Committee (FRAC). The product helps
control the fungal diseases botrytis and powdery mildew in fruits
and vegetables. Demonstrating strong performance in trials across
multiple regions, climates, soil types, production types, pathogen
pressure and crops to date, EVOCA is currently under review by the
U.S. Environmental Protection Agency (EPA) and the Dutch College
voor Toelating van Gewasbeschermingsmiddelen en Biociden (CTGB) in
the European Union for regulatory approval. EVOCA will pave the way
for EVOCA NG, which is expected to be Biotalys’ first commercial
fungicide from its AGROBODY technology platform.
* EVOCA™: Pending Registration. This product
is not currently registered for sale or use in the United States,
the European Union, or elsewhere and is not being offered for
sale.
** Decisions of the CTGB are
subject to appeal during a period of six weeks following the
publication of the decision.
For further information, please
contact:
Toon Musschoot, Head of Investor Relations and Communications
T: +32 (0)9 274 54 00
E: IR@biotalys.com
About Biotalys
Biotalys is an Agricultural Technology (AgTech)
company developing protein-based biocontrol solutions for the
protection of crops and food and aiming to provide alternatives to
conventional chemical pesticides for a more sustainable and safer
food supply. Based on its novel AGROBODY™ technology platform,
Biotalys is developing a strong and diverse pipeline of effective
product candidates with a favorable safety profile that aim to
address key crop pests and diseases across the whole value chain,
from soil to plate. Biotalys was founded in 2013 as a spin-off from
the VIB (Flanders Institute for Biotechnology) and has been listed
on Euronext Brussels since July 2021. The company is based in the
biotech cluster in Ghent, Belgium. More information can be found on
www.biotalys.com.

Important Notice
Biotalys, its business, prospects and financial
position remain exposed and subject to risks and
uncertainties. A description of and reference to these risks
and uncertainties can be found in the annual report on the
consolidated annual accounts published on the company’s
website.
This announcement contains statements which are
"forward-looking statements" or could be considered as such. These
forward-looking statements can be identified by the use of
forward-looking terminology, including the words ‘aim’, 'believe',
'estimate', 'anticipate', 'expect', 'intend', 'may', 'will',
'plan', 'continue', 'ongoing', 'possible', 'predict', 'plans',
'target', 'seek', 'would' or 'should', and contain statements made
by the company regarding the intended results of its strategy. By
their nature, forward-looking statements involve risks and
uncertainties and readers are warned that none of these
forward-looking statements offers any guarantee of future
performance. Biotalys’ actual results may differ materially from
those predicted by the forward-looking statements. Biotalys makes
no undertaking whatsoever to publish updates or adjustments to
these forward-looking statements, unless required to do so by
law.
- Press Release Biotalys receives approval for large scale
demotrials with EVOCA in the Netherlands
- Persbericht Biotalys krijgt goedkeuring voor grootschalige
demonstratieproeven in Nederland


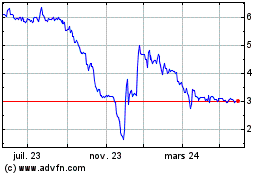
Biotalys (EU:BTLS)
Graphique Historique de l'Action
De Jan 2025 à Fév 2025
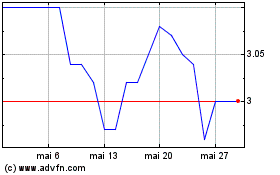
Biotalys (EU:BTLS)
Graphique Historique de l'Action
De Fév 2024 à Fév 2025