0001401914
false
Dare Bioscience, Inc
0001401914
2023-10-12
2023-10-12
iso4217:USD
xbrli:shares
iso4217:USD
xbrli:shares
UNITED
STATES
SECURITIES
AND EXCHANGE COMMISSION
Washington,
D.C. 20549
FORM
8-K
CURRENT
REPORT
Pursuant
to Section 13 or 15(d)
of
the Securities Exchange Act of 1934
Date
of Report (Date of earliest event reported): October 12, 2023
DARÉ
BIOSCIENCE, INC.
(Exact
name of registrant as specified in its charter)
| Delaware |
|
001-36395 |
|
20-4139823 |
(State
or other jurisdiction
of
incorporation) |
|
(Commission
File
Number) |
|
(I.R.S.
Employer
Identification
No.) |
3655
Nobel Drive, Suite 260
San
Diego, CA 92122
(Address
of Principal Executive Offices and Zip Code)
Registrant’s
telephone number, including area code: (858) 926-7655
Not
Applicable
(Former
name or former address, if changed since last report.)
Check
the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under
any of the following provisions (see General Instruction A.2. below):
| ☐ |
Written
communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| |
|
| ☐ |
Soliciting
material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| |
|
| ☐ |
Pre-commencement
communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities
registered pursuant to Section 12(b) of the Act:
| Title
of each class |
|
Trading
Symbol(s) |
|
Name
of each exchange on which registered |
| Common
stock |
|
DARE |
|
Nasdaq
Capital Market |
Indicate
by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405
of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging
growth company ☐
If
an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying
with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item
7.01 |
Regulation
FD Disclosure. |
On
October 16, 2023, Daré Bioscience, Inc. (“Daré”) issued a press release announcing the achievement of the first
commercial milestone under the exclusive license agreement, dated as of March 31, 2022, between Daré and an affiliate of Organon
& Co., Organon International GmbH (“Organon”), as amended (the “License Agreement”). A copy of the press
release is attached as Exhibit 99.1 to this report and is incorporated herein by reference.
Attached
as Exhibit 99.2 to this report is a copy of a presentation about Daré and its product and product candidates, dated October 16,
2023, which is incorporated herein by reference. Daré intends to use the presentation and its contents in various meetings with
investors, securities analysts and others, commencing on October 16, 2023.
The
information in Item 7.01 of this report, including Exhibits 99.1 and 99.2, shall not be deemed “filed” for purposes of Section
18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to liability under that
Section or Sections 11 and 12(a)(2) of the Securities Act of 1933, as amended (the “Securities Act”). The information contained
in this Item 7.01 and in Exhibits 99.1 and 99.2 shall not be incorporated by reference into any filing with the Securities and Exchange
Commission (the “SEC”) made by Daré, whether made before or after the date hereof, regardless of any general incorporation
language in such filing.
On
October 12, 2023, the First Commercial Sale Milestone (as defined in the License Agreement) was achieved in connection with the commercial
launch in the United States of XACIATO™ (clindamycin phosphate) vaginal gel, 2%. As previously reported, this milestone event triggers
a $1.8 million payment from Organon to Daré pursuant to the License Agreement.
| Item
9.01 |
Financial
Statements and Exhibits. |
(d)
Exhibits.
SIGNATURES
Pursuant
to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by
the undersigned hereunto duly authorized.
| |
DARÉ
BIOSCIENCE, INC.
|
| |
|
| Dated:
October 16, 2023 |
By: |
/s/
Sabrina Martucci Johnson |
| |
Name: |
Sabrina
Martucci Johnson |
| |
Title: |
President
and Chief Executive Officer |
Exhibit
99.1
Daré
Bioscience Announces Achievement of First Commercial Milestone Under License Agreement for XACIATO™ (Clindamycin Phosphate) Vaginal
Gel, 2%, FDA-Approved Treatment for Bacterial Vaginosis in Females 12 Years or Older
Daré
to receive $1.8 million payment; eligible for additional potential milestone payments of up to $180 million and tiered double-digit royalties
based on net sales
SAN
DIEGO, October 16, 2023 – Daré Bioscience, Inc. (NASDAQ: DARE), a leader in women’s health innovation, today announced
the first shipment of XACIATOTM
[pronounced zah-she-AH-toe] (clindamycin phosphate) vaginal gel, 2% in connection with the first commercial sale of the product
in the United States, triggering a $1.8 million payment to Daré under its global license agreement with Organon.
“This
is a major achievement for Daré Bioscience, proving our ability to accelerate the development of much-needed, innovative medicines
for women,” said Sabrina Martucci Johnson, President and CEO of Daré Bioscience. “In less than five years since licensing
the technology, we advanced a pivotal clinical trial, gained U.S. Food and Drug Administration approval, and ensured supply to support
launch of XACIATO. We look forward to our continued collaboration with Organon and its established sales force to offer this
important treatment for bacterial vaginosis in females 12 years or older.”
The
New Drug Application for XACIATO was supported by positive results from the DARE-BVFREE Phase 3 randomized, multi-center, double-blinded,
placebo-controlled clinical trial evaluating XACIATO (formerly known as DARE-BV1) for the treatment of bacterial vaginosis (NCT04370548).
Daré
and Organon entered into a global exclusive license agreement for XACIATO in March 2022. Daré received a $10.0 million upfront
payment after the license agreement became effective and a $1.0 million payment in July 2023. In addition to the $1.8 million milestone
payment, Daré is eligible to receive potential future milestone payments of up to $180 million and tiered double-digit royalties
based on net sales.
About
Bacterial Vaginosis
Bacterial
vaginosis is the most common vaginal condition in women of reproductive age in the United States. The condition results from an overgrowth
of certain bacteria, which upsets the balance of the natural vaginal microbiome and can lead to symptoms of odor or discharge. Bacterial
vaginosis may self-resolve in up to 30% of women, but most symptomatic women require treatment. If left untreated, bacterial vaginosis
may lead to serious complications. Bacterial vaginosis has also been shown to disproportionately affect non-Hispanic Black and Mexican
American women.
About
XACIATO
XACIATO
is indicated for the treatment of bacterial vaginosis in females 12 years and older. A single-dose user-filled disposable applicator
delivers 5g of vaginal gel containing 100mg of clindamycin.
Selected
Safety Information
XACIATO
is contraindicated in individuals with a history of hypersensitivity to clindamycin or lincomycin.
Clostridioides
difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including clindamycin, and may range
in severity from mild diarrhea to fatal colitis. Careful medical history is necessary since CDAD has been reported to occur over 2 months
after the administration of antibacterial agents. If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C.
difficile may need to be discontinued.
Polyurethane
condoms are not recommended during treatment with XACIATO or for 7 days following treatment. During this time period, polyurethane condoms
may not be reliable for preventing pregnancy or for protecting against transmission of HIV and other sexually transmitted diseases. Latex
or polyisoprene condoms should be used.
XACIATO
may result in the overgrowth of Candida spp. in the vagina resulting in vulvovaginal candidiasis, which may require antifungal treatment.
The
most common adverse reactions reported in >2% of patients and at a higher rate in the XACIATO group than in the placebo group were
vulvovaginal candidiasis and vulvovaginal discomfort.
XACIATO
has not been studied in pregnant women. However, based on the low systemic absorption of XACIATO following the intravaginal route of
administration in nonpregnant women, maternal use is not likely to result in significant fetal exposure to the drug.
There
are no data on the effect of clindamycin on milk production. The developmental and health benefits of breastfeeding should be considered
along with the mother’s clinical need for clindamycin and any potential adverse effects on the breastfed child from clindamycin
or from the underlying maternal condition.
Please
see the Prescribing Information, Patient Information, and Instructions for Use.
About
Daré Bioscience
Daré
Bioscience is a biopharmaceutical company committed to advancing innovative products for women’s health. The company’s mission
is to identify, develop and bring to market a diverse portfolio of differentiated therapies that prioritize women’s health and
well-being, expand treatment options, and improve outcomes, primarily in the areas of contraception, vaginal health, reproductive health,
menopause, sexual health and fertility.
Daré’s
first FDA-approved product, XACIATO™ (clindamycin phosphate) vaginal gel, 2% is a lincosamide antibacterial indicated for the treatment
of bacterial vaginosis in female patients 12 years of age and older, which is under a global license agreement with Organon. Daré’s
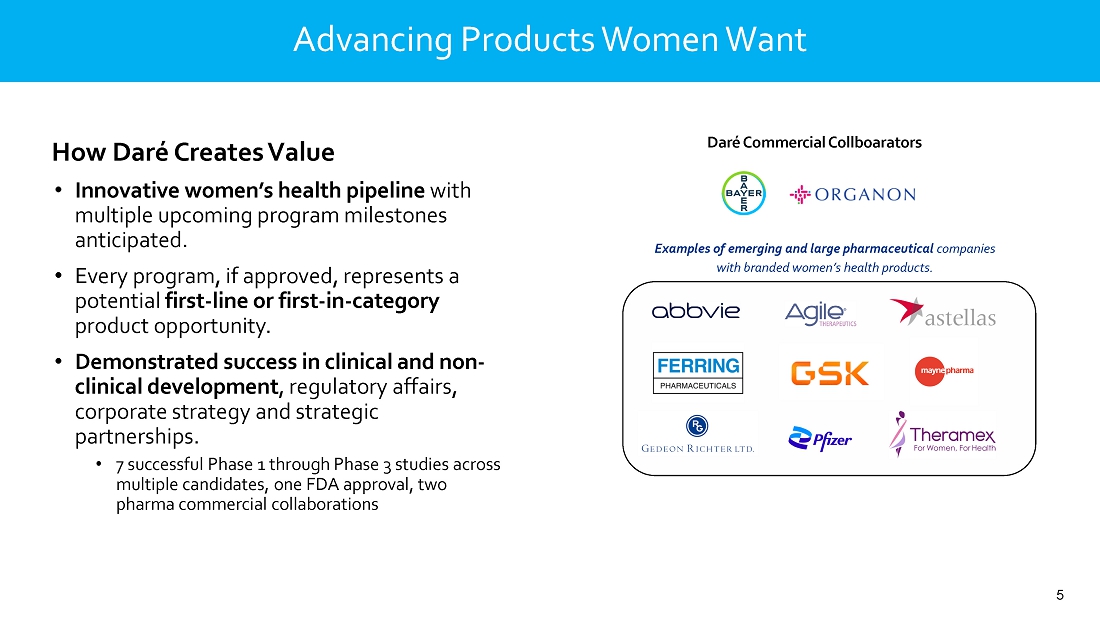
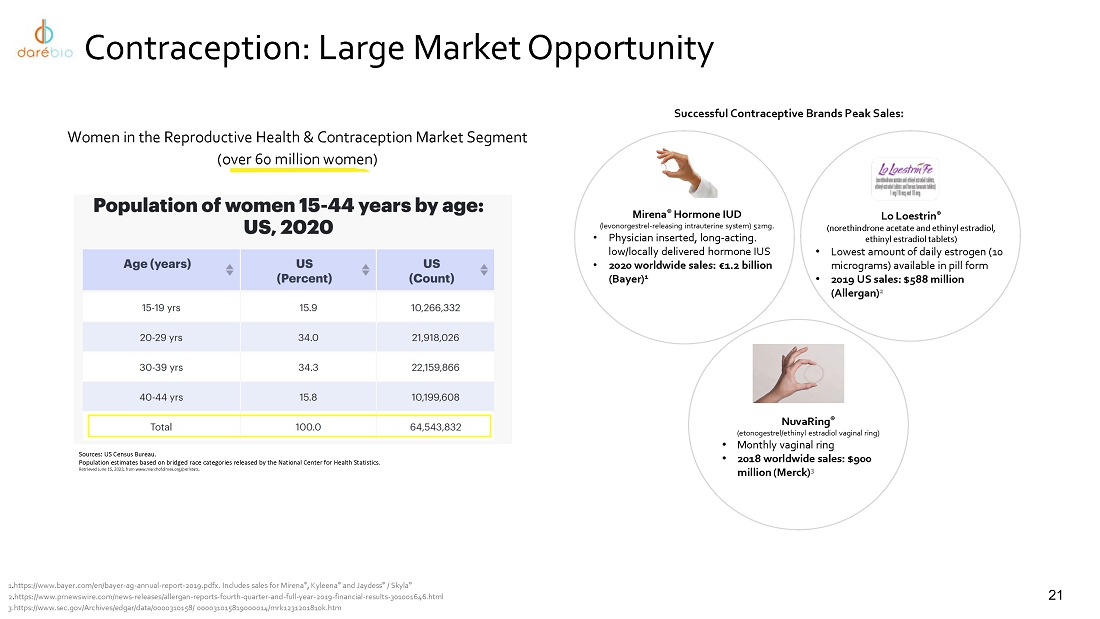
portfolio also includes potential first-in-category candidates in clinical development: Ovaprene®, a novel, hormone-free monthly
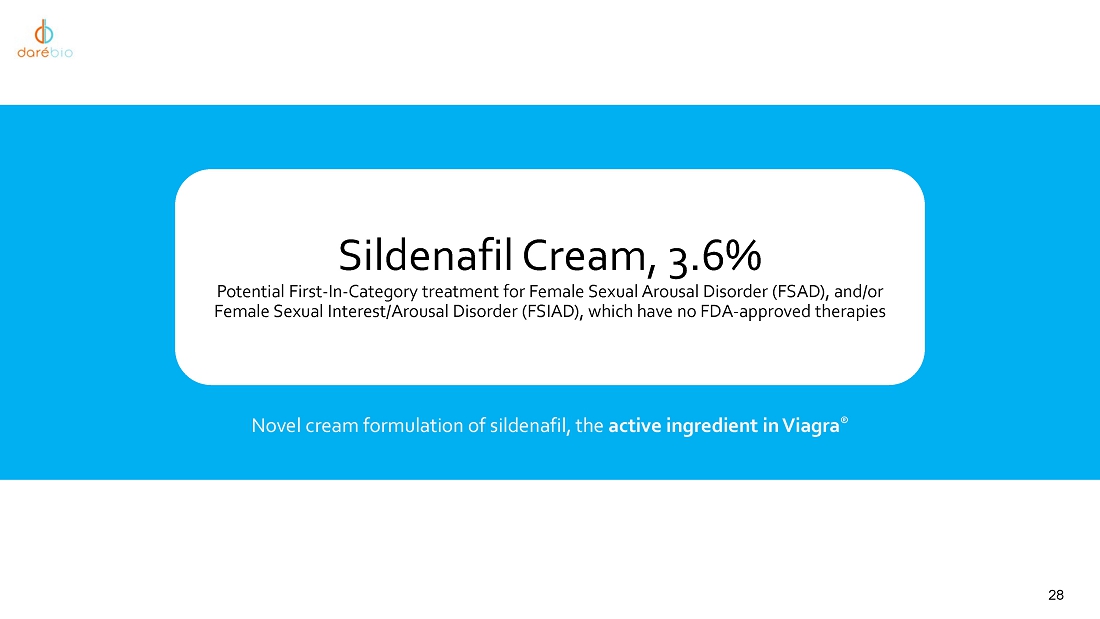
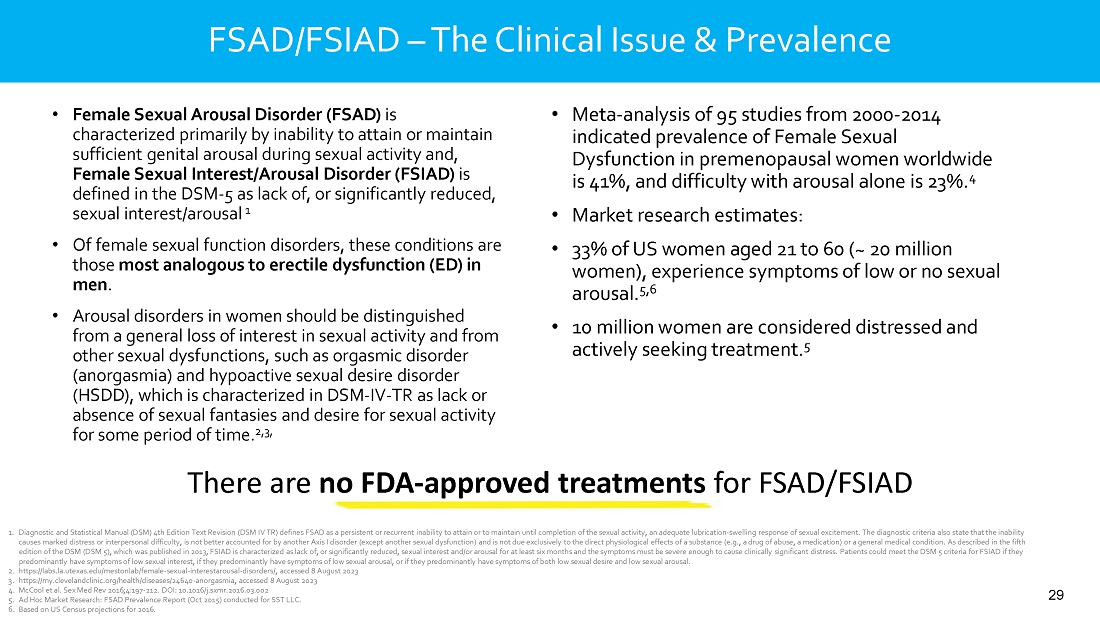
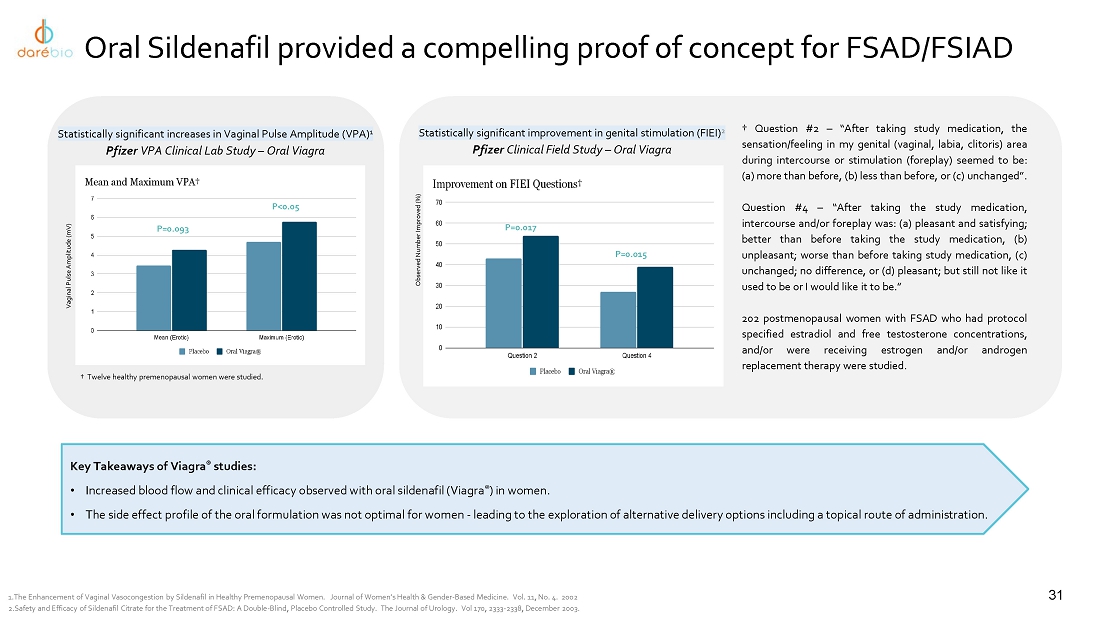
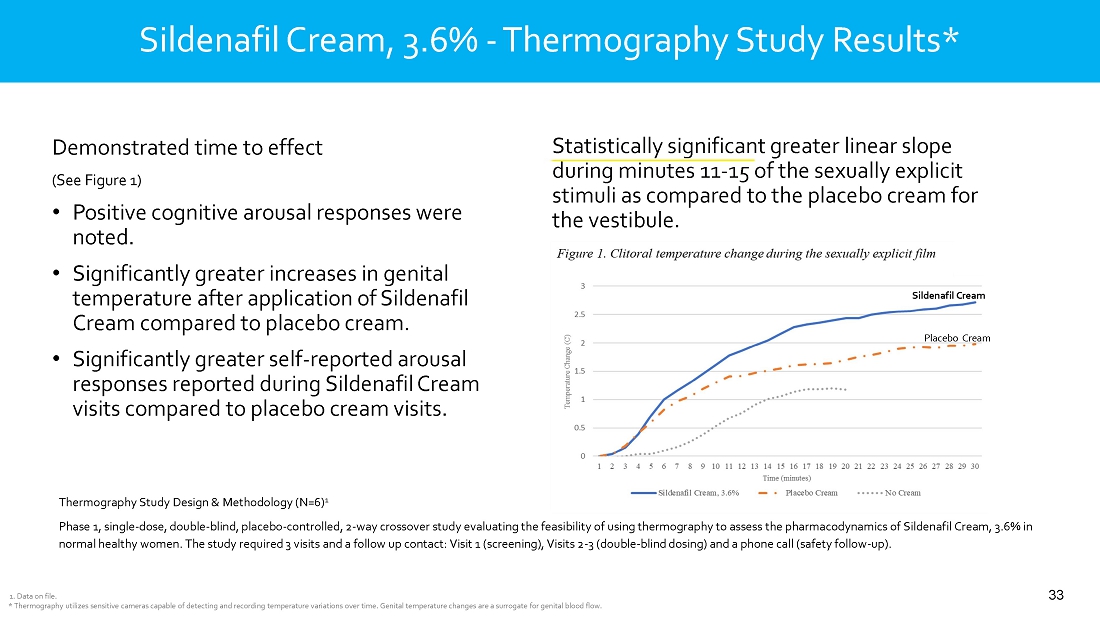
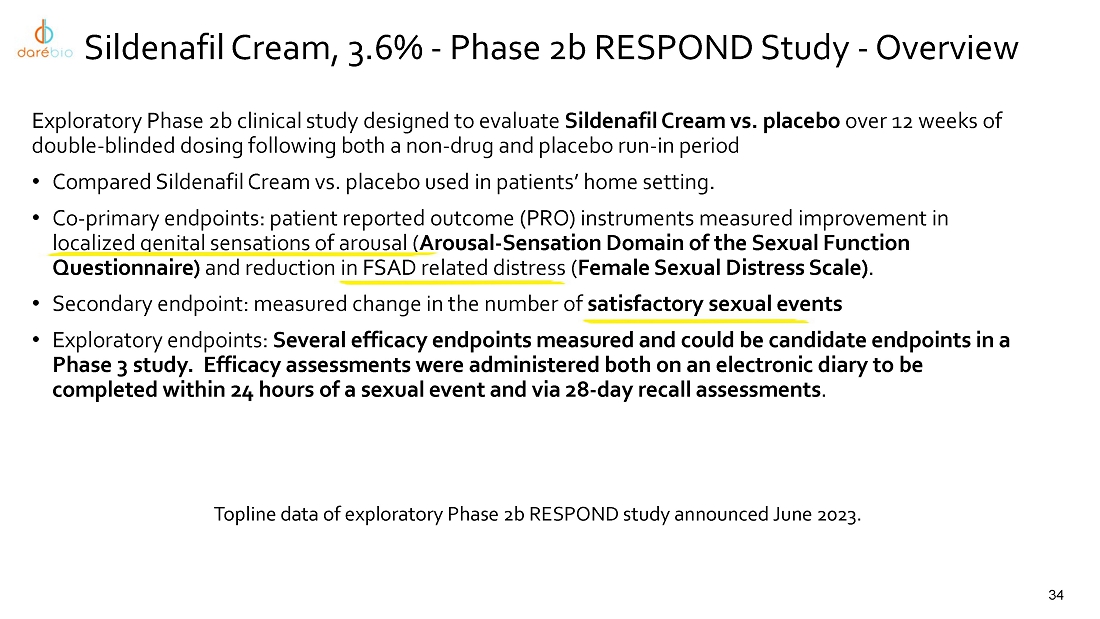
intravaginal contraceptive whose U.S. commercial rights are under a license agreement with Bayer; Sildenafil Cream, 3.6%, a novel cream
formulation of sildenafil to treat female sexual arousal disorder (FSAD) and/or female sexual interest/arousal disorder (FSIAD) utilizing
the active ingredient in Viagra®; and DARE-HRT1, a combination bio-identical estradiol and progesterone intravaginal ring for menopausal
hormone therapy. To learn more about XACIATO, Daré’s full portfolio of women’s health product candidates, and Daré’s
mission to deliver differentiated therapies for women, please visit www.darebioscience.com.
Daré
Bioscience leadership has been named on the Medicine Maker’s Power List and Endpoints News’ Women in Biopharma 2022. In 2023,
Daré’s CEO was honored as one of Fierce Pharma’s Most Influential People in Biopharma for Daré’s contributions
to innovation and advocacy in the women’s health space. Daré Bioscience placed #1 in the Small Company category of the San
Diego Business Journal’s 2023 Best Places to Work Awards.
Daré
may announce material information about its finances, product and product candidates, clinical trials and other matters using the Investors
section of its website (http://ir.darebioscience.com), SEC filings, press releases, public conference calls and webcasts. Daré
will use these channels to distribute material information about the company and may also use social media to communicate important information
about the company, its finances, product and product candidates, clinical trials and other matters. The information Daré posts
on its investor relations website or through social media channels may be deemed to be material information. Daré encourages investors,
the media, and others interested in the company to review the information Daré posts in the Investors section of its website and
to follow these Twitter accounts: @SabrinaDareCEO and @DareBioscience. Any updates to the list of social media channels the company may
use to communicate information will be posted in the Investors section of Daré’s website.
Forward-Looking
Statements
Daré
cautions you that all statements, other than statements of historical facts, contained in this press release, are forward-looking statements.
Forward-looking statements, in some cases, can be identified by terms such as “believe,” “may,” “will,”
“estimate,” “continue,” “anticipate,” “design,” “intend,” “expect,”
“could,” “plan,” “potential,” “predict,” “seek,” “should,” “would,”
“contemplate,” “project,” “target,” “objective,” or the negative version of these words
and similar expressions. In this press release, forward-looking statements include, but are not limited to, statements relating to receipt
of payments under Daré’s license agreement with Organon. Forward-looking statements involve known and unknown risks, uncertainties
and other factors that may cause Daré’s actual results, performance or achievements to be materially different from those
expressed or implied by the forward-looking statements in this press release, including, without limitation: the risks that milestones
under the agreement with Organon may not be achieved and milestone payments received, if any, may be significantly less than the potential
total amount; the potential for royalty payments to be subject to reductions and offsets; the potential for payments under the license
agreement with Organon to become subject to dispute and not received in the amount or on the timeline anticipated; dependence on Organon
to commercialize licensed products and on other third parties for commercial supplies of licensed products and components and Daré’s
lack of control over the efforts and resources expended by those third parties; Daré’s ability to raise additional capital
when and as needed to support its operations, execute its business strategy and continue as a going concern; general industry conditions
and competition; the effects of macroeconomic conditions such as inflation, rising interest rates and geopolitical events on Daré’s
operations, financial results and condition, and ability to achieve current plans and objectives; the impact of pharmaceutical industry
regulation and health care legislation in the United States and internationally; the risk that developments by competitors make Daré’s
product or product candidates less competitive or obsolete; difficulties establishing and sustaining relationships with development and/or
commercial collaborators; failure of Daré’s product or product candidates, if approved, to gain market acceptance or obtain
adequate coverage or reimbursement from third-party payers; Daré’s ability to retain its licensed rights to develop and
commercialize a product or product candidate; Daré’s ability to satisfy the monetary obligations and other requirements
in connection with its exclusive, in-license agreements covering the critical patents and related intellectual property related to its
product and product candidates; Daré’s ability to adequately protect or enforce its, or its licensor’s, intellectual
property rights; the lack of patent protection for the active ingredients in certain of Daré’s product candidates which
could expose its products to competition from other formulations using the same active ingredients; product liability claims; governmental
investigations or actions relating to Daré’s product or product candidates or the business activities of Daré, its
commercial collaborators or other third parties on which Daré relies; the impact of pharmaceutical industry regulation and health
care legislation in the United States and internationally; global trends toward health care cost containment; cybersecurity incidents
or similar events that compromise Daré’s technology systems or those of third parties on which it relies and/or significantly
disrupt Daré’s business; and disputes or other developments concerning Daré’s intellectual property rights.
Daré’s forward-looking statements are based upon its current expectations and involve assumptions that may never materialize
or may prove to be incorrect. All forward-looking statements are expressly qualified in their entirety by these cautionary statements.
For a detailed description of Daré’s risks and uncertainties, you are encouraged to review its documents filed with the
SEC including Daré’s recent filings on Form 8-K, Form 10-K and Form 10-Q. You are cautioned not to place undue reliance
on forward-looking statements, which speak only as of the date on which they were made. Daré undertakes no obligation to update
such statements to reflect events that occur or circumstances that exist after the date on which they were made, except as required by
law.
Contacts:
Media
and Investors on behalf of Daré Bioscience, Inc:
Camilla
White / Simona Kormanikova
Dentons
Global Advisors
DareBioscience@dentonsglobaladvisors.com
/ 1.212.466.6450
Source:
Daré Bioscience, Inc.
Exhibit
99.2




































v3.23.3
Cover
|
Oct. 12, 2023 |
| Cover [Abstract] |
|
| Document Type |
8-K
|
| Amendment Flag |
false
|
| Document Period End Date |
Oct. 12, 2023
|
| Entity File Number |
001-36395
|
| Entity Registrant Name |
Dare Bioscience, Inc
|
| Entity Central Index Key |
0001401914
|
| Entity Tax Identification Number |
20-4139823
|
| Entity Incorporation, State or Country Code |
DE
|
| Entity Address, Address Line One |
3655
Nobel Drive
|
| Entity Address, Address Line Two |
Suite 260
|
| Entity Address, City or Town |
San
Diego
|
| Entity Address, State or Province |
CA
|
| Entity Address, Postal Zip Code |
92122
|
| City Area Code |
(858)
|
| Local Phone Number |
926-7655
|
| Written Communications |
false
|
| Soliciting Material |
false
|
| Pre-commencement Tender Offer |
false
|
| Pre-commencement Issuer Tender Offer |
false
|
| Title of 12(b) Security |
Common
stock
|
| Trading Symbol |
DARE
|
| Security Exchange Name |
NASDAQ
|
| Entity Emerging Growth Company |
false
|
| Entity Information, Former Legal or Registered Name |
Not
Applicable
|
| X |
- DefinitionBoolean flag that is true when the XBRL content amends previously-filed or accepted submission.
| Name: |
dei_AmendmentFlag |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionFor the EDGAR submission types of Form 8-K: the date of the report, the date of the earliest event reported; for the EDGAR submission types of Form N-1A: the filing date; for all other submission types: the end of the reporting or transition period. The format of the date is YYYY-MM-DD.
| Name: |
dei_DocumentPeriodEndDate |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:dateItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe type of document being provided (such as 10-K, 10-Q, 485BPOS, etc). The document type is limited to the same value as the supporting SEC submission type, or the word 'Other'.
| Name: |
dei_DocumentType |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:submissionTypeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 1 such as Attn, Building Name, Street Name
| Name: |
dei_EntityAddressAddressLine1 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionAddress Line 2 such as Street or Suite number
| Name: |
dei_EntityAddressAddressLine2 |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- Definition
+ References
+ Details
| Name: |
dei_EntityAddressCityOrTown |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCode for the postal or zip code
| Name: |
dei_EntityAddressPostalZipCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the state or province.
| Name: |
dei_EntityAddressStateOrProvince |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:stateOrProvinceItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionA unique 10-digit SEC-issued value to identify entities that have filed disclosures with the SEC. It is commonly abbreviated as CIK. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityCentralIndexKey |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:centralIndexKeyItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionIndicate if registrant meets the emerging growth company criteria. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityEmergingGrowthCompany |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionCommission file number. The field allows up to 17 characters. The prefix may contain 1-3 digits, the sequence number may contain 1-8 digits, the optional suffix may contain 1-4 characters, and the fields are separated with a hyphen.
| Name: |
dei_EntityFileNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:fileNumberItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTwo-character EDGAR code representing the state or country of incorporation.
| Name: |
dei_EntityIncorporationStateCountryCode |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarStateCountryItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe exact name of the entity filing the report as specified in its charter, which is required by forms filed with the SEC. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityRegistrantName |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionThe Tax Identification Number (TIN), also known as an Employer Identification Number (EIN), is a unique 9-digit value assigned by the IRS. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b-2
| Name: |
dei_EntityTaxIdentificationNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:employerIdItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionLocal phone number for entity.
| Name: |
dei_LocalPhoneNumber |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:normalizedStringItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 13e
-Subsection 4c
| Name: |
dei_PreCommencementIssuerTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 14d
-Subsection 2b
| Name: |
dei_PreCommencementTenderOffer |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTitle of a 12(b) registered security. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection b
| Name: |
dei_Security12bTitle |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:securityTitleItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionName of the Exchange on which a security is registered. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Number 240
-Section 12
-Subsection d1-1
| Name: |
dei_SecurityExchangeName |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:edgarExchangeCodeItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as soliciting material pursuant to Rule 14a-12 under the Exchange Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Exchange Act
-Section 14a
-Number 240
-Subsection 12
| Name: |
dei_SolicitingMaterial |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionTrading symbol of an instrument as listed on an exchange.
| Name: |
dei_TradingSymbol |
| Namespace Prefix: |
dei_ |
| Data Type: |
dei:tradingSymbolItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
| X |
- DefinitionBoolean flag that is true when the Form 8-K filing is intended to satisfy the filing obligation of the registrant as written communications pursuant to Rule 425 under the Securities Act. Reference 1: http://www.xbrl.org/2003/role/presentationRef
-Publisher SEC
-Name Securities Act
-Number 230
-Section 425
| Name: |
dei_WrittenCommunications |
| Namespace Prefix: |
dei_ |
| Data Type: |
xbrli:booleanItemType |
| Balance Type: |
na |
| Period Type: |
duration |
|
Dare Bioscience (NASDAQ:DARE)
Graphique Historique de l'Action
De Déc 2024 à Jan 2025

Dare Bioscience (NASDAQ:DARE)
Graphique Historique de l'Action
De Jan 2024 à Jan 2025
