Aileron Therapeutics to Host Virtual KOL Investor Event on May 19, 2022
13 Mai 2022 - 1:00PM

Aileron Therapeutics (Nasdaq: ALRN), a chemoprotection oncology
company that aspires to make chemotherapy safer and thereby more
effective to save more patients’ lives, today announced that it
will host a virtual KOL investor event on Thursday, May 19, 2022,
at 4:00 pm ET/ 1:00 pm PT.
The virtual event will feature presentations
from the company's President and Chief Executive Officer, Manuel
Aivado, M.D., Ph.D., as well as key opinion leaders, Ralf Paus,
M.D., DSc, FRSB, Professor of Dermatology, Dr. Phillip Frost
Department of Dermatology & Cutaneous Surgery, University of
Miami Miller School of Medicine and Eric Anderson, M.D., Ph.D.,
Medical Oncologist, Assistant Professor of Medicine, School of
Medicine Oregon Health & Science University.
The presentations will highlight several topics
related to ALRN-6924’s revolutionary potential as the first
precision medicine-based supportive care drug, and the current
supportive care landscape and unmet need associated with
chemotherapy-induced toxicities, including:
- New non-clinical data that will be
presented in a late-breaking oral presentation at the Society for
Clinical Dermatology 2022 Annual Meeting on May 19, 2022,
demonstrating ALRN-6924 protected human hair follicles and their
stem cells from chemotherapy-induced acute and permanent
damage;
- An overview of Aileron’s ongoing Phase 1b randomized,
double-blind, placebo-controlled clinical trial of patients with
p53-mutated advanced non-small cell lung cancer, including the
trial design and its composite endpoint;
- An introduction to Aileron’s
upcoming Phase 1b randomized clinical trial of patients with
p53-mutated ER+/HER2- neoadjuvant breast cancer, which is on track
to initiate in 2Q 2022, and an update on the ongoing Phase 1
pharmacology study in healthy human volunteers; and
- A review of the company’s planned
data readouts in 2022
A live webcast of the event will be available on
the "Events & Presentations" in the Investors & Media
section of the company's website at investors.aileronrx.com. A
replay of the webcast will be available for 30 days following the
presentation.
About Aileron
TherapeuticsAileron is a clinical stage chemoprotection
oncology company that aspires to make chemotherapy safer and
thereby more effective to save more patients’ lives. ALRN-6924, our
first-in-class MDM2/MDMX dual inhibitor, is designed to activate
p53, which in turn upregulates p21, a known inhibitor of the cell
replication cycle. ALRN-6924 is the only reported chemoprotective
agent in clinical development to employ a biomarker strategy, in
which we exclusively focus on treating patients with p53-mutated
cancers. Our targeted strategy is designed to selectively protect
multiple healthy cell types throughout the body from chemotherapy
without protecting cancer cells. As a result, healthy cells are
spared from chemotherapeutic destruction while chemotherapy
continues to kill cancer cells. By reducing or eliminating multiple
chemotherapy-induced side effects, ALRN-6924 may improve patients’
quality of life and help them better tolerate chemotherapy.
Enhanced tolerability may result in fewer dose reductions or delays
of chemotherapy and the potential for improved efficacy.
Forward-Looking
StatementsStatements in this press release about Aileron’s
future expectations, plans and prospects, as well as any other
statements regarding matters that are not historical facts, may
constitute forward-looking statements within the meaning of The
Private Securities Litigation Reform Act of 1995. These statements
include, but are not limited to, statements about the potential of
ALRN-6924 as a chemoprotective agent, the Company’s strategy and
clinical development plans. The words “anticipate,” “believe,”
“continue,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,”
“potential,” “predict,” “project,” “should,” “target,” “would” and
similar expressions are intended to identify forward-looking
statements, although not all forward-looking statements contain
these identifying words. Actual results may differ materially from
those indicated by such forward-looking statements as a result of
various important factors, including whether Aileron’s cash
resources will be sufficient to fund its continuing operations for
the periods anticipated or with respect to the matters anticipated;
whether initial results of clinical trials will be indicative of
final results of those trials or results obtained in future
clinical trials, including trials in different indications; whether
ALRN-6924 will advance through the clinical trial process on a
timely basis, or at all; whether the results of such trials will be
accepted by and warrant submission for approval from the United
States Food and Drug Administration or equivalent foreign
regulatory agencies; whether ALRN-6924 will receive approval from
regulatory agencies on a timely basis or at all or in which
territories or indications ALRN-6924 may receive approval; whether,
if ALRN-6924 obtains approval, it will be successfully distributed
and marketed; what impact the coronavirus pandemic may have on the
timing of our clinical development, clinical supply and our
operations; and other factors discussed in the “Risk Factors”
section of Aileron’s annual report on Form 10-K for the year ended
December 31, 2021, filed on March 28, 2022, and risks described in
other filings that Aileron may make with the Securities and
Exchange Commission. Any forward-looking statements contained in
this press release speak only as of the date hereof, and Aileron
specifically disclaims any obligation to update any forward-looking
statement, whether because of new information, future events or
otherwise.
| Investor Contact: |
Media Contact: |
| Stern Investor Relations |
Liz Melone |
| Alexander Lobo |
617-256-6622 |
| alex.lobo@sternir.com |
lmelone@aileronrx.com |
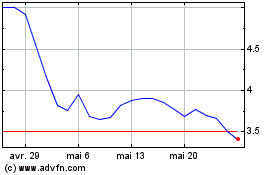
Aileron Therapeutics (NASDAQ:ALRN)
Graphique Historique de l'Action
De Jan 2025 à Fév 2025
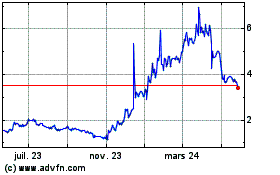
Aileron Therapeutics (NASDAQ:ALRN)
Graphique Historique de l'Action
De Fév 2024 à Fév 2025